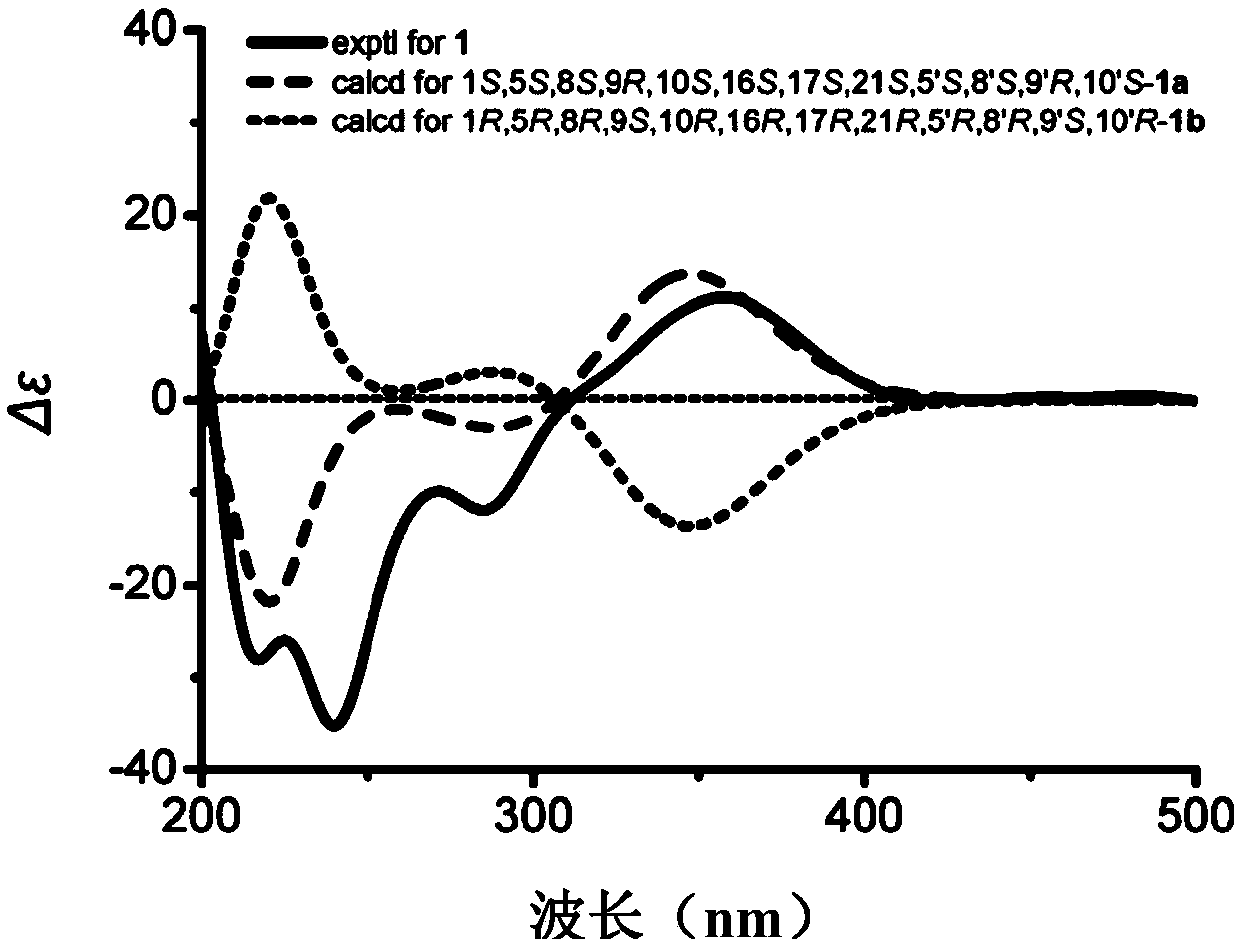
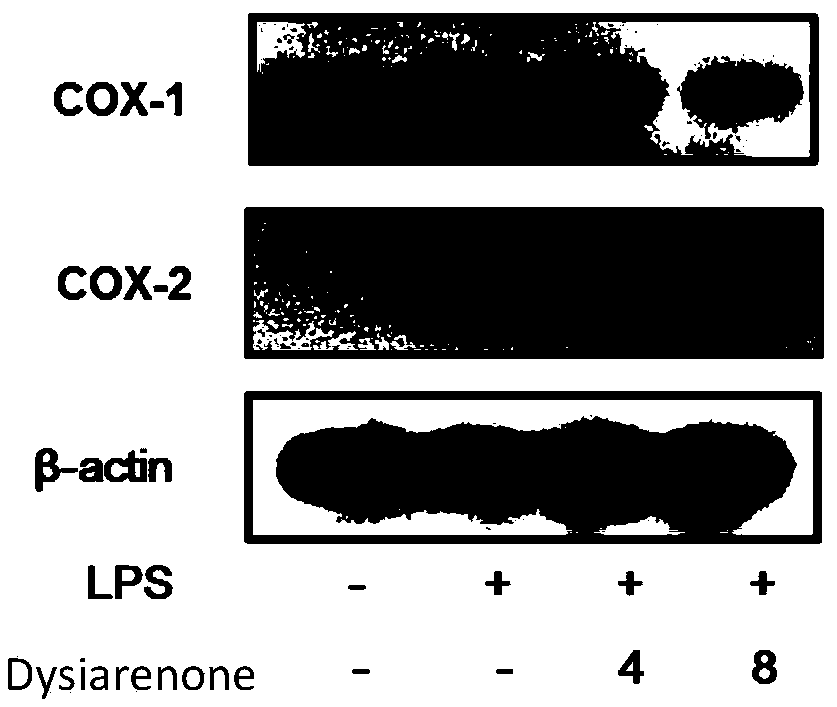
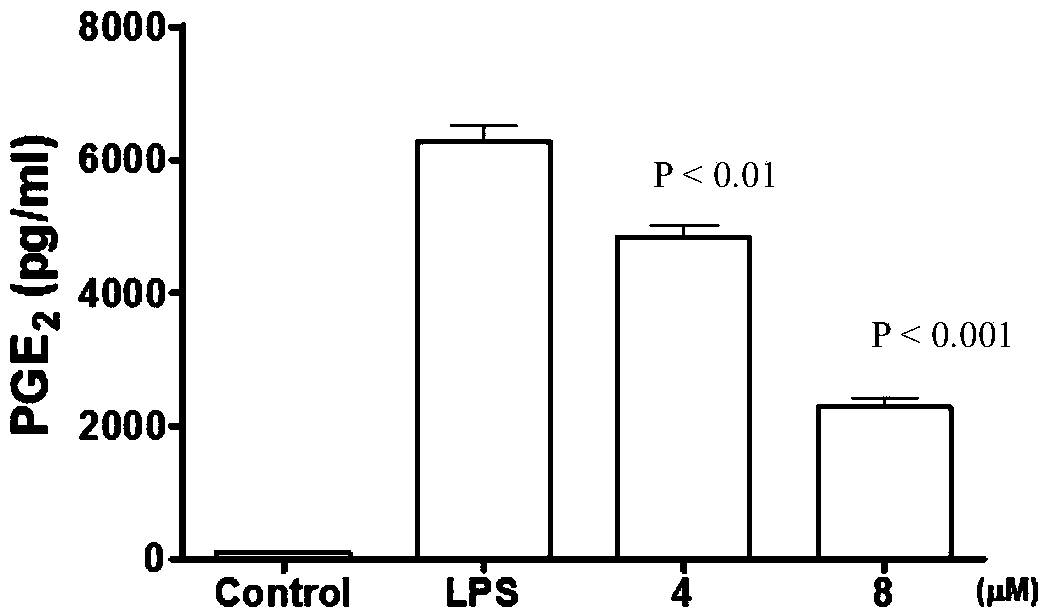
Sponge-derived meroterpenoid compound Dysiarenone as well as preparation method and application thereof
A compound and source technology, applied in the field of medicine, can solve the problems of no heteroterpene compounds, etc., and achieve the effect of significant release inhibitory activity, simple preparation method, and strong inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 compound dysiarenone
[0036] Get pre-frozen and chopped sponge (wet weight 520g), extract 8 times with 95% ethanol (1L) cold soaking respectively, each time for a week, combine the extracts, and concentrate the extracts under reduced pressure to obtain the total extract. The extract was suspended in water, and extracted 5 times with an equal volume of dichloromethane. The extracts were combined and concentrated under reduced pressure to obtain a dichloromethane extract (2.7 g).
[0037] The above-mentioned dichloromethane extract (2.7g) was separated by silica gel column chromatography under reduced pressure, and was eluted with petroleum ether-ethyl acetate gradient. According to the results of thin-layer chromatography TLC analysis, a fraction containing sesquiterpene quinone compounds was obtained, and then This cut is further enriched with SephadexLH-20 gel column, adopts sherwood oil-methylene chloride-methanol (4:1:5) system, obtai...
Embodiment 2
[0044] Example 2 Inhibition of lipopolysaccharide (LPS)-induced release of PGE from RAW 264.7 mouse macrophages in vitro 2 experiment
[0045] The compounds of the present invention, dysiarenone, avarol and dexamethasone (Dexamethasone, a synthetic anti-inflammatory drug) were used as samples to inhibit the release of PGE from RAW 264.7 mouse macrophages induced by lipopolysaccharide in vitro 2 In the experiment, a parallel experiment of a blank group (control) without adding samples was carried out at the same time. The sample was dissolved in DMSO and stored at low temperature, and the concentration of DMSO in the final system was controlled within the range that did not affect the detection activity. Three replicate wells were set up for each sample concentration in the test.
[0046] Specific experimental steps:
[0047] Raw 264.7 mouse macrophages purchased from Shanghai Institute of Biological Sciences were cultured in RPMI 1640 (including 10% (v / v) FBS, 100 U / mL peni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com