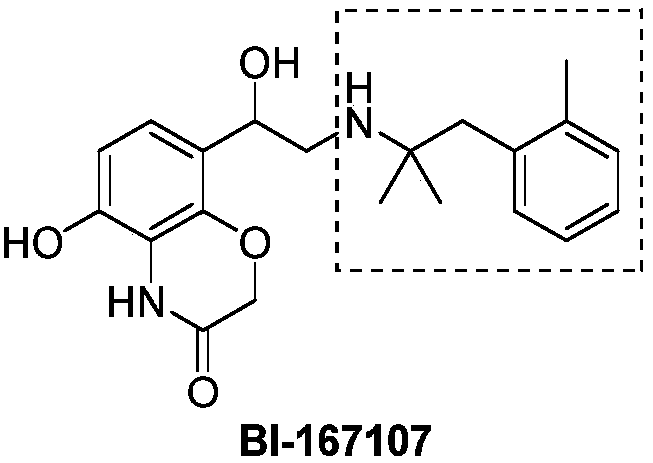
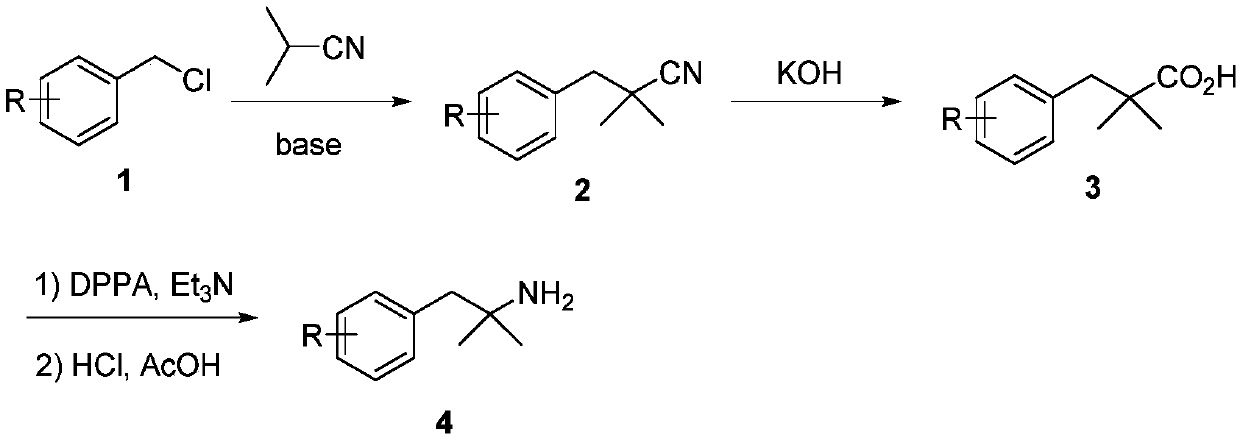
Novel synthesis method of 2-methyl-1-substituted phenyl-2-propylamine compounds
A compound, a new synthesis technology, applied in the preparation of organic compounds, chemical instruments and methods, preparation of functional groups substituted with amino groups, etc., can solve problems such as unusable, and achieve the effect of simple operation, simplified reaction route, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
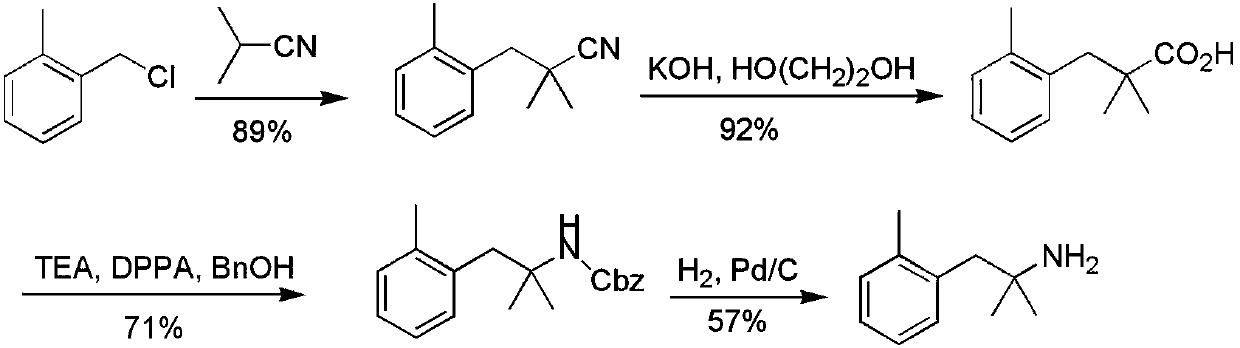
[0022] Preparation of 2-methyl-1-(2-methylphenyl)-2-propanamine (4a; R=2-Me)
[0023] Step 1: Preparation of 2-methyl-1-(2-methylphenyl)-2-butyronitrile (2a; R=2-Me)
Embodiment 1
[0024] Example 1: Dissolve diisopropylamine (1.88mL, 13.4mmol, 1.1eq) in 10mL of tetrahydrofuran, ice-bath to -78°C, and 2 Under protection, n-butyllithium (2.8mL, 7.0mmol, 1.1eq) was added dropwise, and after stirring for 30min, light yellow LDA was prepared. Isobutyronitrile (2.39mL, 26.6mmol, 1.1eq) was added dropwise to the reaction, stirred for 1 hour, then 2-methylbenzyl chloride (1.5g, 10.6mmol) was added, and the reaction was stopped after stirring for 25min. Add 10mL of 10% hydrochloric acid and 30ml of water to the mixture to quench the reaction, extract 3 times with 20mL of ethyl acetate, wash 3 times with 15mL of water and 3 times with 15mL of saturated brine, pour the organic phase Put it into a clean Erlenmeyer flask, add an appropriate amount of anhydrous sodium sulfate to dry for half an hour. After purification by column chromatography, a pale yellow liquid was obtained with a yield of 98%. 1 H NMR (300MHz, CDCl 3 ): δ7.30-7.16 (m, 4H), 2.88 (s, 2H), 2.38 (...
Embodiment 2
[0026] Example 2: Take 2-methyl-1-(2-methylphenyl)-2-butyronitrile 2a (1.4g, 8.1mmol) and add it to a 50mL single-necked flask, then add KOH (1.13g, 20.2mmol, 2.5eq) and ethylene glycol (15mL), heated to reflux at 175°C for 6 hours and stopped the reaction, then added a 10% hydrochloric acid solution to the reaction solution to adjust the pH=1; first extracted 3 times with 20mL of ethyl acetate ; then extracted 3 times with 15mL of water, washed three times with 15mL of saturated brine; the organic phase was poured into a clean Erlenmeyer flask, dried over anhydrous sodium sulfate, and purified by column chromatography to obtain a brown liquid with a yield of 89% . 1 H NMR (300MHz, CDCl 3 ): δ7.17-7.12(m, 4H), 2.97(s, 2H), 2.33(s, 3H), 1.24(d, J=9.6Hz, 6H).
[0027] Step 3: 2-Methyl-1-(2-methylphenyl)-2-propanamine (4a; R=2-Me)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com