Use of peptidomimetic compounds in the preparation of drugs for inhibiting intracellular growth of Chlamydia trachomatis
A technology of Chlamydia trachomatis and peptide compounds, which can be applied in the directions of antibacterial drugs, drug combinations, peptides, etc., can solve the problem of not inhibiting Chlamydia trachomatis and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
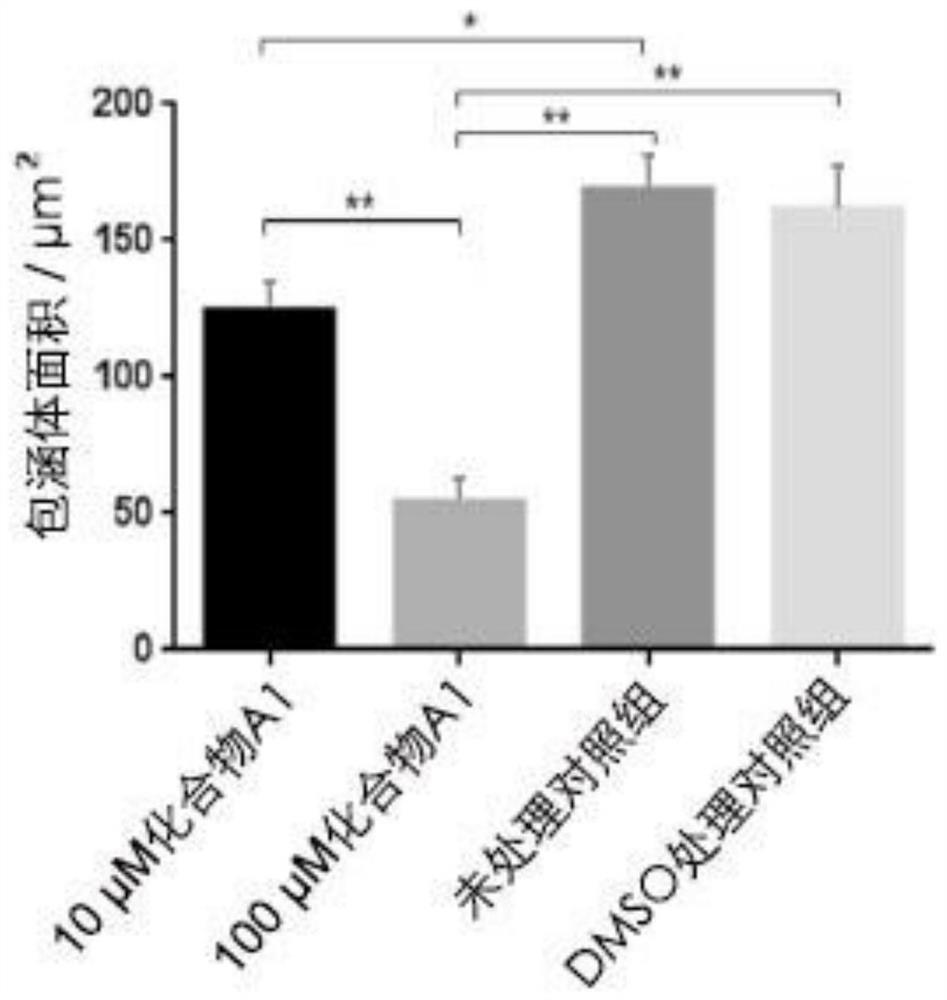
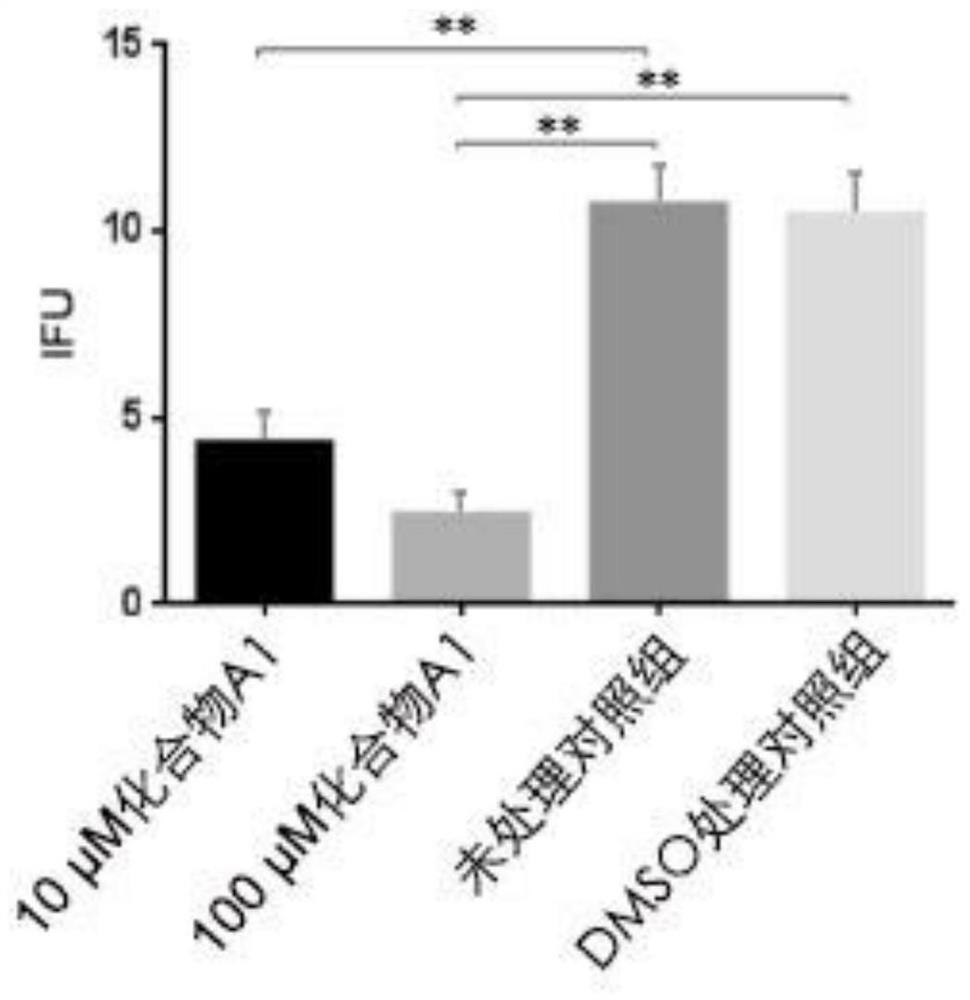
[0028] The effect of peptidomimetic compound A1 on inhibiting the reproduction of Chlamydia trachomatis. HeLa229 with good logarithmic phase growth was added to each well at 1.5×10 5 A number of cell plates, placed at 37 ° C, 5% CO 2 After routine culture in the cell culture box for 24 hours, wash twice with PBS, add 1 mL of 1640 medium containing 10% fetal bovine serum, and add Chlamydia trachomatis liquid and complete medium at 1:200, 1:400, 1:600, respectively. The protoplasma of Chlamydia trachomatis was added at a ratio of 1:800 and 1:1000, and Hoechst staining was carried out after 24 hours. Randomly select 3 fields of view to count cells and inclusion bodies, and obtain IFU (Inclusion-forming unit, inclusion body forming unit) and MOI (multiplicity of infection, infection copy number, MOI=IFU / cell number), which are used to optimize the optimal detection rate of Chlamydia trachomatis. optimal infectious dose. Furthermore, after infecting HeLa229 cells with L2 serotyp...
Embodiment 2
[0032]The peptidomimetic compound A1 inhibits the normal inclusion body formation of Chlamydia trachomatis.
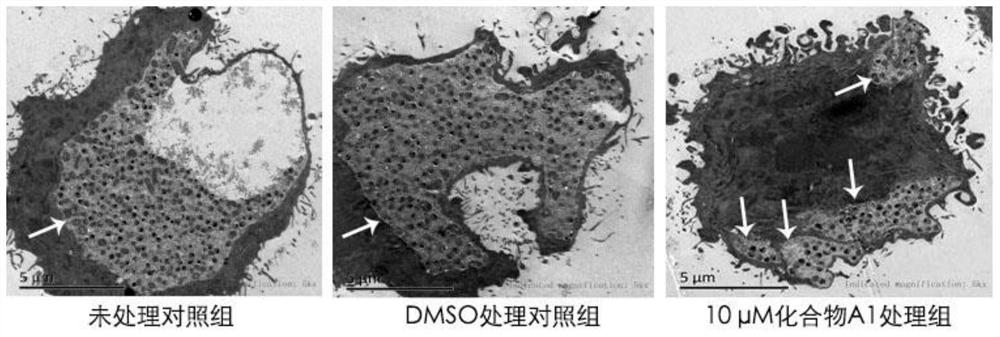
[0033] image 3 It is an electron micrograph of Chlamydia trachomatis infecting Hela229 cells with compound A1. image 3 It can be seen that the inhibitor of Chlamydia trachomatis HtrA protein causes small inclusion bodies to fail to fuse into large inclusion bodies, and after Chlamydia trachomatis infected Hela229 cells were treated with 10 μM compound A1, there were 4 small inclusion bodies.
[0034] Cytotoxicity test of peptidomimetic compound A1. 5 × 10 3 The number of Hella229 cells was seeded into a 96-well plate, and the cells were placed at 37°C, 5% CO 2 After routine culture in the cell culture box for 24 hours, a control group (Hela229 cells + normal medium), A1 concentration 100 μM + Hela229 cells, and DMSO + Hela229 cell groups were set. Three replicate wells were set up in each group, and the total volume of each well was 100 μL. After 20 h of culture,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com