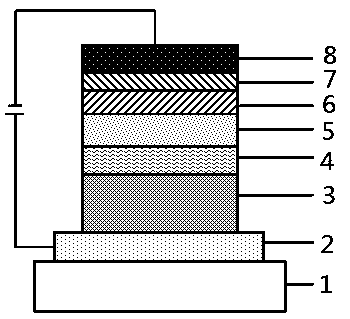
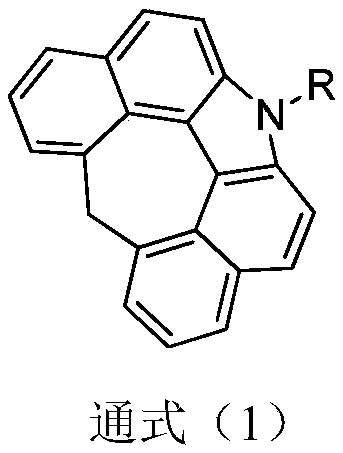
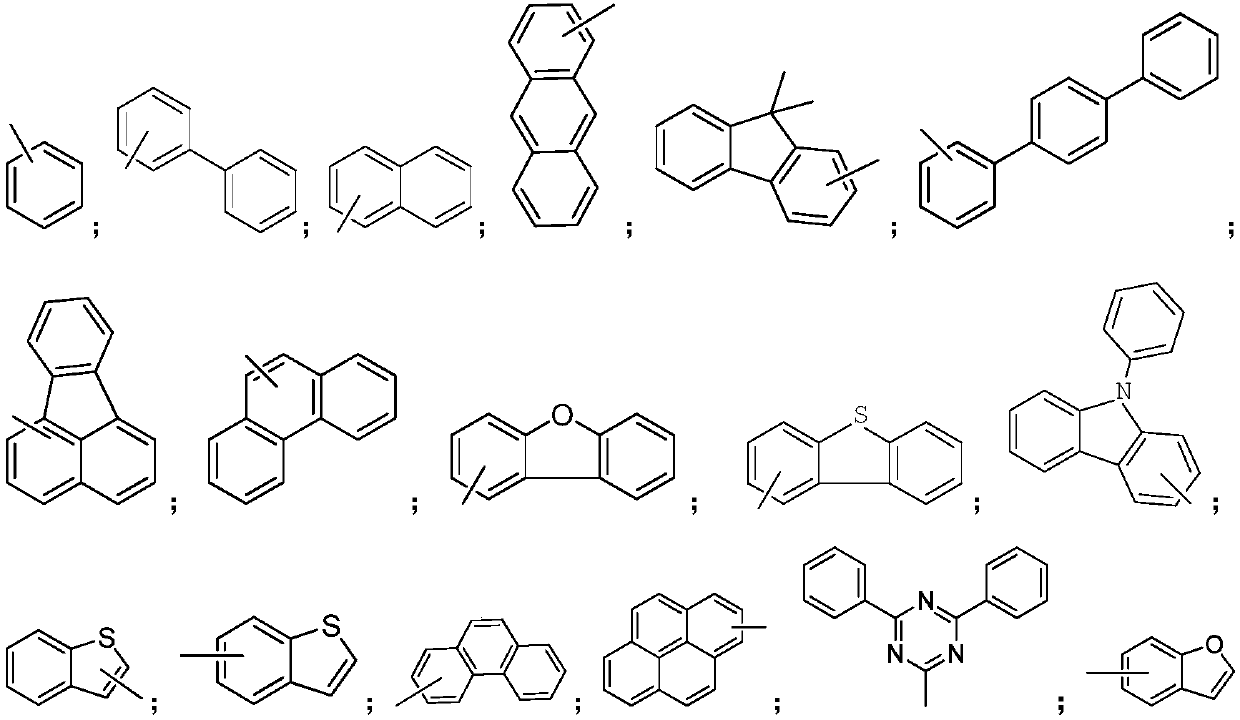
Preparation method for organic compound by using seven-element ring as core structure and application thereof on OLED
A technology of organic compounds and core structures, applied in the field of organic optoelectronic materials, can solve the problems of shifting emission wavelengths, unsuitable for large-scale production, and difficult to guarantee the stability of devices, and achieve the effect of high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the synthesis of compound 1
[0025]
[0026] The specific synthetic route of compound 1:
[0027]
[0028] Under the protection of nitrogen, to a 500ml three-necked flask, add 27.9g compound A (100mmol), 17.3g compound B (110mmol), 3.8g cuprous iodide (20mmol), 42.4g tripotassium phosphate (200mmol), 200g di Toluene was heated to reflux for 7 hours, and no compound A was traced by TLC. After the reaction is complete, cool down to 30°C, filter, rinse with 40ml*3 xylene, wash the filtrate with water 150g*4 to pH=7, and pass through a silica gel column under normal pressure after drying. After passing through the column, wash the silica gel column with 40ml*2 xylene. After rinsing, combine the eluent and the passing liquid to remove the solvent under reduced pressure. Afterwards, 21.8 g of compound 1 was obtained, yield: 63.6%, HPLC: 99.25%.
[0029] HPLC-MS: The theoretical molecular weight of compound 1 is 355.4, and the actual detection result mo...
Embodiment 2
[0030] Embodiment 2: the synthesis of compound 5
[0031]
[0032] Synthetic route of compound 5
[0033]
[0034] Under the protection of nitrogen, to a 500ml three-necked flask, add 27.9g compound A (100mmol), 22.8g compound C (110mmol), 2.9g cuprous bromide (20mmol), 42.4g tripotassium phosphate (200mmol), 200g di Toluene was heated to reflux for 6 hours, and no compound A was traced by TLC. After the reaction is complete, cool down to 30°C, filter, rinse with 40ml*3 xylene, wash the filtrate with water 150g*4 to pH=7, and pass through a silica gel column under normal pressure after drying. After passing through the column, rinse the silica gel column with 40ml*2 xylene. After rinsing, combine the eluent and the column liquid to remove the solvent under reduced pressure. After removing the solvent, add 180g of toluene and 150g of ethanol to recrystallize three times 31.7 g of compound 1 was obtained, yield: 78.0%, HPLC: 99.78%.
[0035] HPLC-MS: The theoretical mol...
Embodiment 3
[0036] Embodiment 3: the synthesis of compound 22
[0037]
[0038] Synthetic route of compound 22
[0039]
[0040] Under the protection of nitrogen, to a 500ml three-necked flask, add 27.9g compound A (100mmol), 30.9g compound D (110mmol), 7.6g cuprous iodide (40mmol), 27.6g potassium carbonate (200mmol), 200g o-di Chlorobenzene, heated to reflux for 6 hours, traced by TLC to no compound A. After the reaction is complete, cool down to 30°C, filter, rinse with 40ml*3 toluene, wash the filtrate with 150g*3 water until pH=7, remove the solvent, add 250g toluene to dissolve, pass through a silica gel column under normal pressure, and pass through the column, 50ml toluene *2 Rinse the silica gel column. After rinsing, combine the eluent and the column solution to remove the solvent under reduced pressure. After desolventization, add 230g ethyl acetate and 230g petroleum ether to recrystallize twice. After drying, 33.5g of compound 22 is obtained. , Yield: 69.7%, HPLC: 99....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com