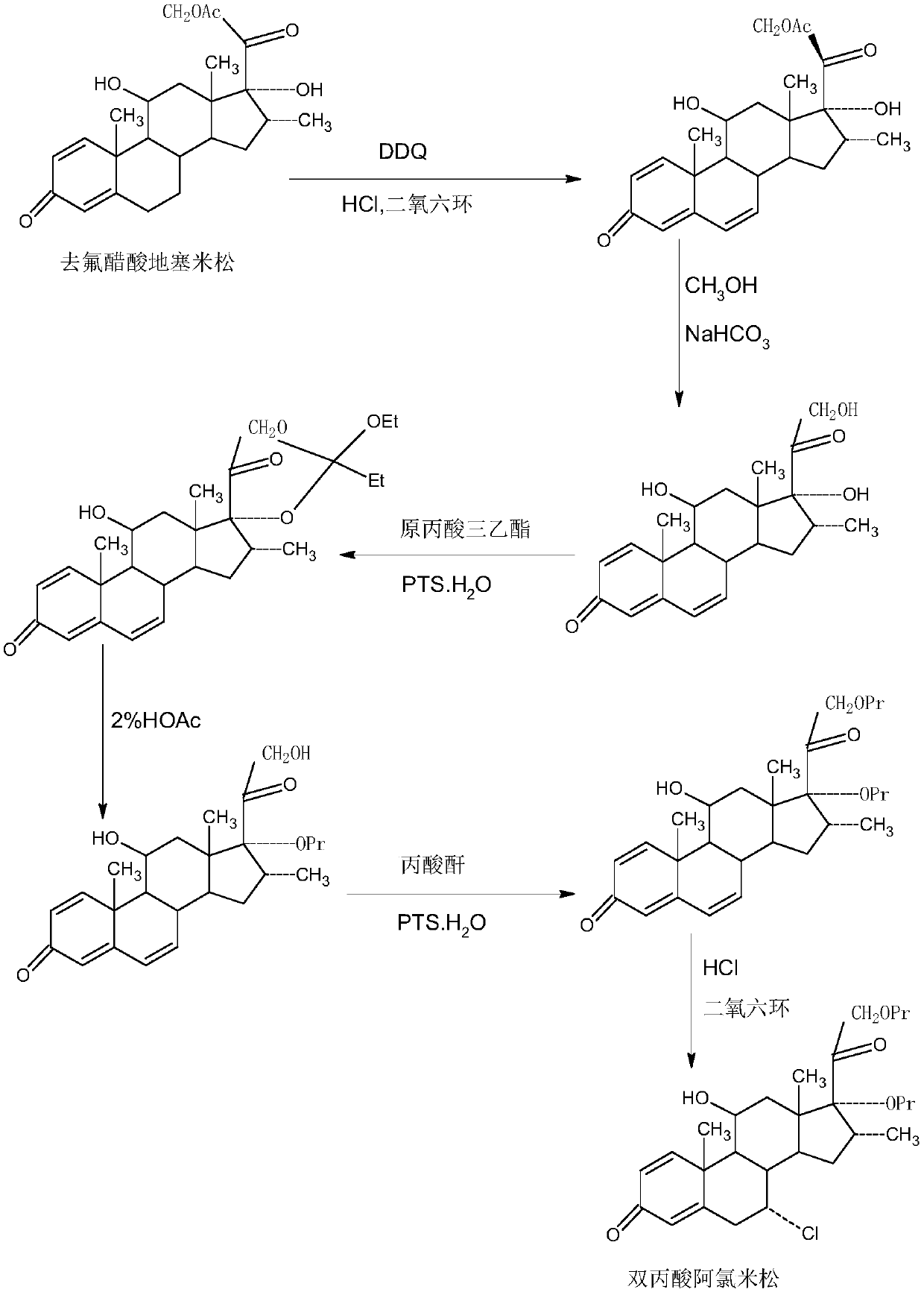
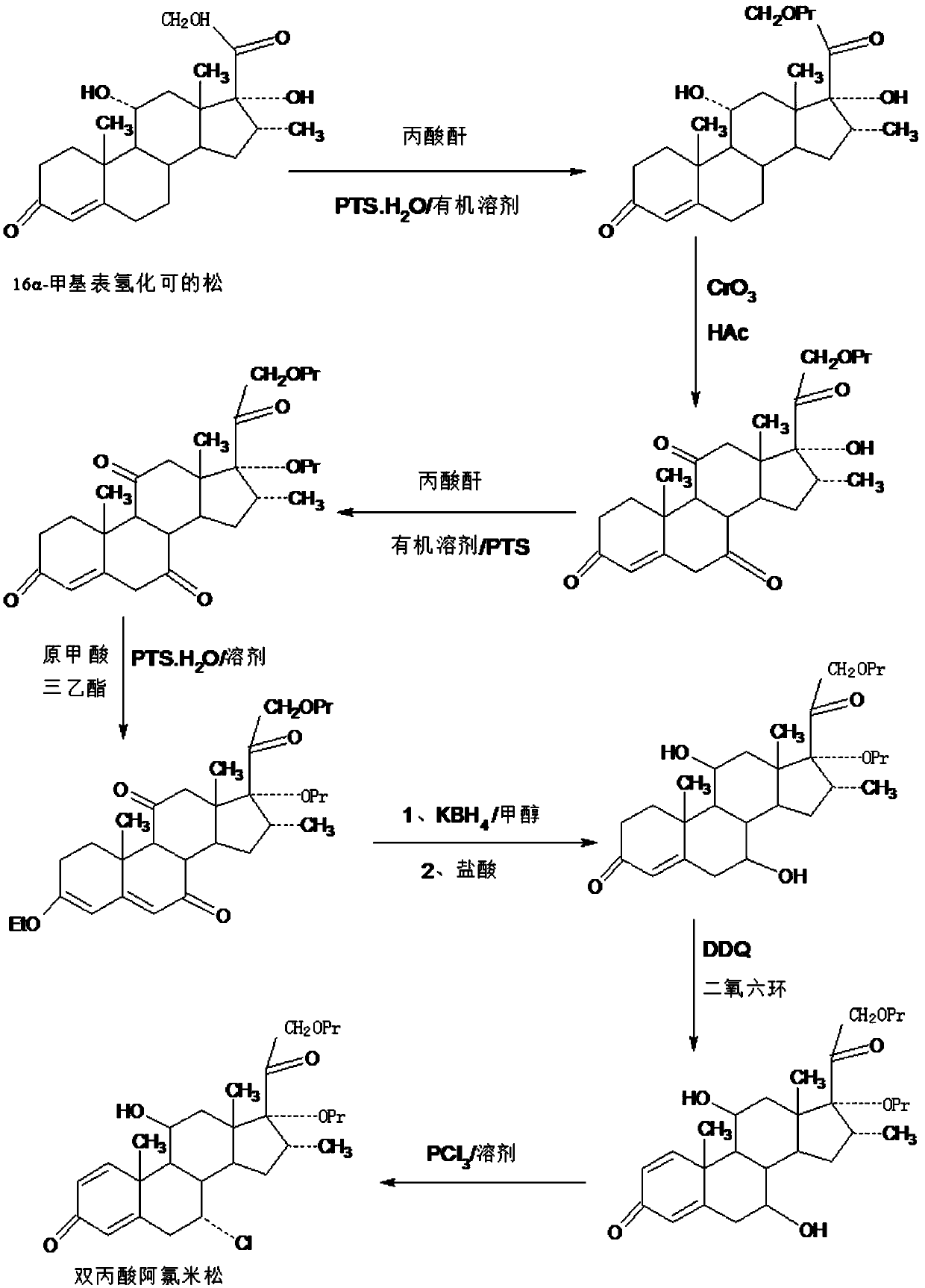
Method for preparing reduced intermediate product for aclomethasone dipropionate
A technology of aclomethasone dipropionate and an intermediate is applied in the field of reduced intermediate products for preparing aclomethasone dipropionate, and can solve the problems of long synthesis route, low synthesis total yield, many side reactions, and the like, To achieve the effect of simple production operation, economical and environmentally friendly process, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A. Preparation of esterified product: In a 1000ml three-necked flask, add 100g16a-methyl epihydrocortisone, 200ml propionic acid, 40g propionic anhydride, 5g p-toluenesulfonic acid, react at 20~25°C for 6~8 hours, TLC confirms the end point of the reaction. After the reaction is completed, slowly add the reaction solution dropwise to a 5000ml three-neck flask containing 2000ml molar concentration of 5% sodium hydroxide solution, stir and crystallize at 0~5°C for 4~5 hours, filter, The filtrate is discharged into the wastewater treatment tank, and the filter cake is added to 600ml of ethanol aqueous solution with a molar concentration of 20%, stirred at 30-35°C for 5-6 hours, then cooled to 0-5°C, stirred and crystallized for 5-6 hours, filtered , the filtrate was used in the next batch of refining process, the filter cake was washed and dried to obtain the esterified product: 112g of 16a-methyl epihydrocortisone-21 propionate, the HPLC content was 97.4%, and the weight y...
Embodiment 2
[0052] A. Preparation of esterified product: In a 1000ml three-neck flask, add 100g16a-methyl epihydrocortisone, 500ml chloroform, 40g propionic anhydride, 8g p-toluenesulfonic acid, react at 20~25°C for 6~8 hours, TLC Confirm the end point of the reaction. After the reaction is completed, slowly add 20ml of sodium hydroxide solution with a molar concentration of 50% dropwise. After water separation, concentrate under reduced pressure to recover 90-95% chloroform, then add 500ml of pure water, and stir at 0-5°C. Crystallize for 4-5 hours, filter, and discharge the filtrate into the wastewater treatment tank, add the filter cake to 600ml of ethanol aqueous solution with a molar concentration of 20%, stir at 30-35°C for 5-6 hours, then cool down to 0-5°C and stir Crystallize for 5-6 hours, filter, and use the filtrate in the next batch of refining process, wash the filter cake, and dry to obtain esterified product: 109.5 g of 16a-methyl-epihydrocortisone-21 propionate, HPLC conte...
Embodiment 3
[0060] A. Preparation of esterified product: In a 1000ml three-necked flask, add 100g16a-methyl epihydrocortisone, 500ml toluene, 40g propionic anhydride, 6g trifluoroacetic acid, react at 20~25°C for 6~8 hours, confirm by TLC At the end of the reaction, after the reaction, slowly add 20ml of sodium hydroxide solution with a molar concentration of 50% dropwise, after water separation, concentrate under reduced pressure to recover 90-95% toluene, then add 500ml of pure water, stir and crystallize at 0-5°C After 4 to 5 hours, filter, and the filtrate is discharged into the waste water treatment tank, and the filter cake is added to 600ml of ethanol aqueous solution with a molar concentration of 20%, stirred at 30 to 35°C for 5 to 6 hours, then cooled to 0 to 5°C, stirred and analyzed. crystallized for 5-6 hours, filtered, and the filtrate was used in the next batch of refining process, the filter cake was washed and dried to obtain esterified product: 106.8g of 16a-methyl-epihydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com