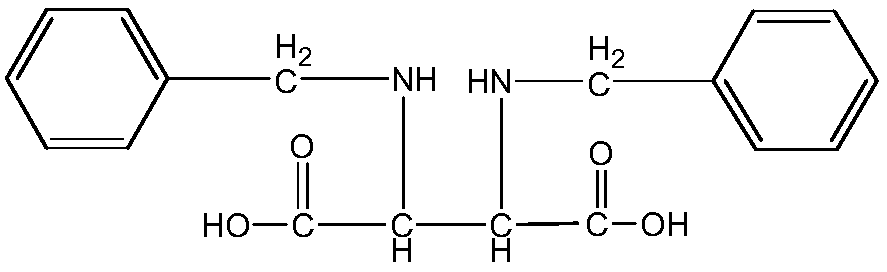
Method for preparing 1,3-bisbenzyl-2-imidazolidine-4,5-dicarboxylic acid
A technology of dibenzylimidazole and dibenzylaminosuccinic acid, applied in the field of chemical synthesis, can solve problems such as personal safety threats and environmental hazards, and achieve the effects of avoiding environmental hazards, less side reactions, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] This example provides a method for preparing 1,3-dibenzylimidazol-2-one-4,5-dicarboxylic acid, comprising the following steps:
[0025] Add 25kg of cis-2,3-dibenzylaminosuccinic acid to 20kg of dimethyl carbonate by weight, and then add 3kg of zinc acetate, and react at a temperature of 130°C and a pressure of 2Mpa for 6h.
[0026] After the reaction, the mixed solution was filtered at normal temperature and pressure, and the filtered filter cake was first washed twice with 3% HCl aqueous solution at 0°C, and then washed once with 0°C ice water. After washing, the filter cake was vacuum-dried , That is, 1,3-dibenzyl imidazol-2-one-4,5-dicarboxylic acid.
[0027] The yield of 1,3-dibenzylimidazol-2-one-4,5-dicarboxylic acid prepared by the method described in this example was 91%, and the purity was 98%.
Embodiment 2
[0029] This example provides a method for preparing 1,3-dibenzylimidazol-2-one-4,5-dicarboxylic acid, comprising the following steps:
[0030] Add 20kg of cis-2,3-dibenzylaminosuccinic acid to 25kg of dimethyl carbonate, then add 2kg of zinc acetate, and react at a temperature of 140°C and a pressure of 3Mpa for 7h.
[0031] After the reaction, the mixed solution was filtered at normal temperature and pressure, and the filtered filter cake was first washed twice with 3% HCl aqueous solution at 0°C, and then washed once with 0°C ice water. After washing, the filter cake was vacuum-dried , That is, 1,3-dibenzyl imidazol-2-one-4,5-dicarboxylic acid.
[0032] The yield of 1,3 dibenzyl imidazol-2-one-4,5 dicarboxylic acid in the method described in this example is 89%, and the purity is 98.5%.
Embodiment 3
[0034] This example provides a method for preparing 1,3 dibenzyl imidazol-2-one-4,5 dicarboxylic acid, comprising the following steps:
[0035] Add 30kg of cis-2,3-dibenzylaminosuccinic acid to 30kg of dimethyl carbonate, then add 3kg of zinc acetate, and react at a temperature of 150°C and a pressure of 2Mpa for 7h.
[0036] After the reaction, the mixed solution was filtered at normal temperature and pressure, and the filtered filter cake was first washed twice with 3% HCl aqueous solution at 0°C, and then washed once with 0°C ice water. After washing, the filter cake was vacuum-dried , That is, 1,3-dibenzyl imidazol-2-one-4,5-dicarboxylic acid.
[0037] The production yield of 1,3-dibenzylimidazol-2-one-4,5-dicarboxylic acid by the method described in this example is 94%, and the purity is 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com