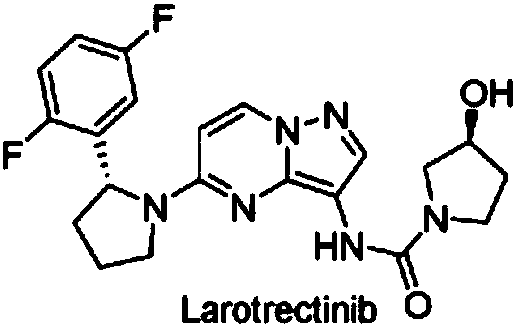
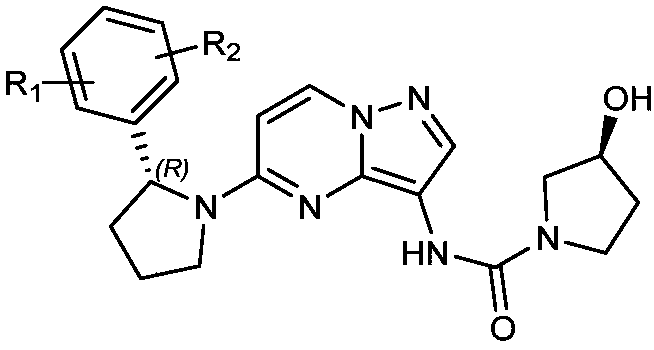
Radioactive iodine marked Larotrectinib compound, and preparation method and application thereof
A technology of radioactive iodine and compounds, applied in the direction of in vivo radioactive preparations, radioactive carriers, organic chemical methods, etc., can solve problems such as lack of technical means
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
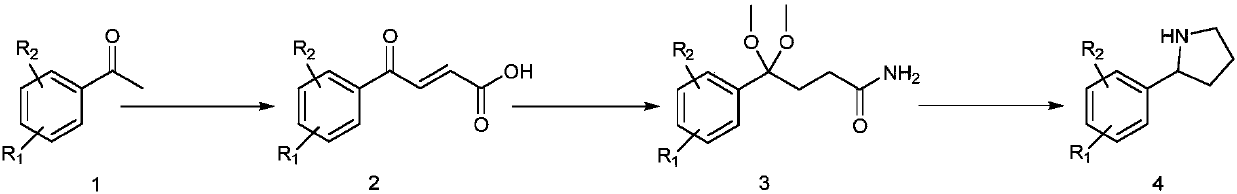
[0193] Embodiment one: the synthesis of key intermediate 4: the synthesis of 2-(5-fluoro-2-iodophenyl)pyrrolidine
Embodiment 1a
[0194] Embodiment 1a, the synthesis of (E)-4-(5-fluoro-2-iodophenyl)-4-oxobut-2-enoic acid
[0195]
[0196] In a 500mL reactor, add 5-fluoro-2-iodoacetophenone (39.6g, 150mmol), glyoxylic acid monohydrate (13.9g, 151mmol), heat the reaction and evaporate water under reduced pressure (95 degrees, 0.1Mpa ), reacted for 3 hours, cooled the reaction mixture to room temperature, added 5% potassium carbonate aqueous solution (300mL), extracted 2 times with ethyl acetate, 200mL each time, after the aqueous layer was acidified (10% hydrochloric acid, 300mL), extracted with ethyl acetate 2 times, 200 mL each time, combined the organic phases, washed with brine, dried over anhydrous sodium sulfate, and removed the solvent under reduced pressure to obtain an orange solid. The solid was dissolved in glacial acetic acid (50mL), concentrated hydrochloric acid (36%, 5mL), the mixture was heated to reflux for 4 hours, the acetic acid was removed under reduced pressure, the residue was ext...
Embodiment 1b
[0199] Synthesis of embodiment 1b, 4-(5-fluoro-2-iodophenyl)-4-oxobutanoic acid
[0200]
[0201] In a 500mL reaction flask, add 210mL of acetic acid, 75mL of water, starting material (47.1g, 147mmol), and under stirring, add zinc powder (10.9g, 166mmol) in batches to the reaction mixture, add it in about 1 hour, and the mixture Stirring was continued for 3 hours, the reactant was filtered, the filter cake was rinsed with ethyl acetate (300mL), the organic phase was washed 3 times with brine, each 100mL, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain the target product (29.8g, 63%). M.P.152 degrees.
[0202] 1 H NMR (400MHz, CDCl 3 )12.00(1H,brs),7.91-7.71(3H,m),3.23(2H,t,J=6.26),2.57(2H,t,J=6.24).
[0203] MS (EI) m / z 322 (M+).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com