Detection test paper for quickly diagnosing Lyme disease, and preparation method thereof
A technology for rapid diagnosis and detection of test strips, applied in the field of immunodiagnosis, can solve the problems of low sensitivity, long detection time, lack of specificity, etc., and achieve the effects of high detection accuracy, short detection time and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Such as figure 1 Shown, Example 1: Preparation of Lyme Disease Recombinant Antigen
[0025] (1) Expression of Lyme disease recombinant antigens
[0026] In NCBI, look for protein groups containing Lyme major antigen epitopes, BmpA (NCBI Reference Sequence: WP_002656850.1), OspC (NCBI Reference Sequence: WP_010890595.1) and VlsE (GenBank: ACC99642.1) to analyze the above three proteins Antigen epitope, the most dominant antigen epitope was selected for gene recombination expression, since the target recombinant protein needs to be expressed in E. The protein was successfully expressed in high yield and high solubility.
[0027] After the codon-optimized gene recombination sequence was double-digested with BamHI and SalI restriction endonucleases (purchased from NEB Company, namely NewEngland Biolabs), it was inserted into pET30a treated with the same two restriction enzymes (Novagen product, Cat. No. 69909 In -3), the Lyme disease recombinant expression vector plasmid...
Embodiment 2
[0030] Example 2: Preparation of Lyme disease recombinant antigen-colloidal gold conjugate
[0031] (1) Preparation of colloidal gold
[0032] Dilute 1% chloroauric acid to 0.01% (mass percentage content) with ultrapure water, add in a 100ml Erlenmeyer flask, and heat to boil with a heating magnetic stirrer. Then accurately absorb 2.0ml of 1% trisodium citrate and slowly add it to the conical flask, stir evenly, continue heating until the solution turns from black to gray and then red, and add an appropriate amount of 0.02% NaN 3 After stirring evenly, the colloidal gold solution was obtained and stored at 4°C. The newly prepared qualified colloidal gold solution should be a solution with a pure, stable, transparent appearance and no sediment and floating matter. After the preparation is completed, it is observed under an electron microscope, and colloidal gold particles with a suitable diameter are selected.
[0033] (2) Preparation of markers
[0034] Under the dark stir...
Embodiment 3
[0035] Example 3: Preparation of Colloidal Gold Immunochromatography Test Paper for Rapid Diagnosis of Lyme Disease
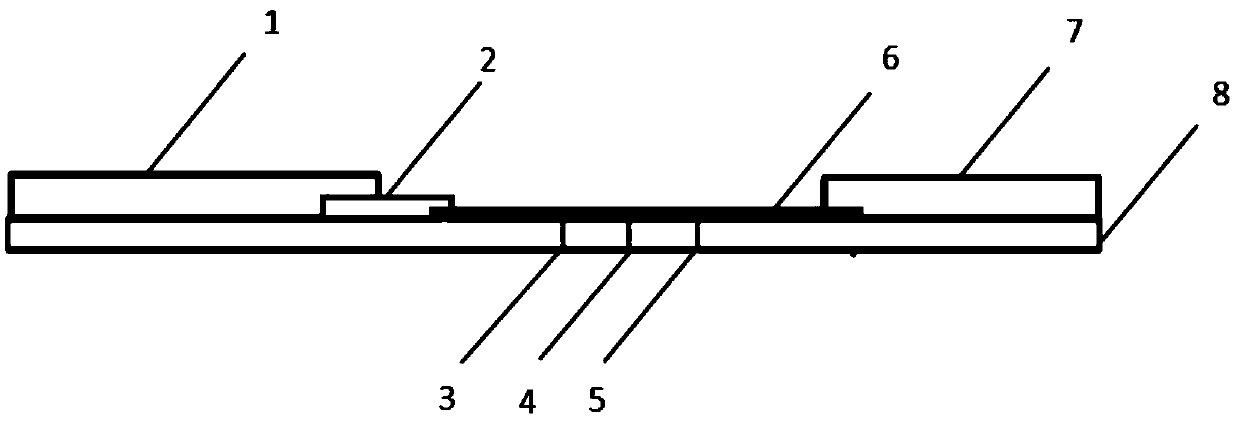
[0036] Such as figure 1 As shown, a colloidal gold immunochromatographic test paper for rapid diagnosis of Lyme disease includes a bottom plate 8 , a sample pad 1 , a gold standard pad 2 , a nitrocellulose membrane 6 and an absorbent pad 7 . The sample pad 1 , the gold standard pad 2 , the nitrocellulose membrane 6 , and the water-absorbing pad 7 are arranged in sequence and bonded together, and are all arranged on the bottom plate 8 . The nitrocellulose membrane 6 is provided with a first detection line 3 , a second detection line 4 and a quality control line 5 . Mouse anti-human IgM monoclonal antibody is set at the first detection line 3 . Mouse anti-human IgG monoclonal antibody is set at the second detection line 4 . Goat anti-mouse IgG polyclonal antibody is set at quality control line 5. The Lyme disease recombinant antigen-colloidal gold conjugate p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com