Oxygen evolution catalyst with low noble metal loading amount for water electrolysis unit
A water electrolyzer and precious metal technology, applied in the field of water electrolysis, can solve the problems such as the inability to greatly reduce the catalyst precious metal content, low precious metal loading, poor electronic conductivity, etc., and achieve improved binding force and electron transfer rate, high electrocatalytic activity. , the effect of strengthening electronic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
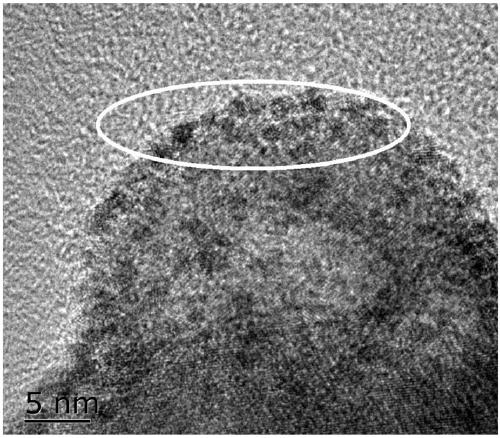
Image
Examples
Embodiment 1
[0030] A preparation method for an iridium-titanium composite catalyst, comprising the steps of:
[0031] (1) Get 360mg of chloroiridic acid (mass fraction of iridium in chloroiridic acid is 35%) and add 120mL of pure water, ultrasonic 10min is prepared as the chloroiridic acid solution that iridium concentration is 1mg / mL, adding concentration is 0.1mol / L Sodium hydroxide solution, adjust the pH to about 6, then add 1.08g of powdered titanium with a particle size of 20-200nm, ultrasonically disperse for 10min, and then stir at 25°C for 1h to obtain a mixed solution;
[0032] (2) Nitrogen gas is passed through the mixed solution obtained in step (1) to remove dissolved oxygen, then it is placed in an ice-water bath, and a sodium hydroxide solution with a concentration of 1mol / L is added under stirring conditions, wherein sodium hydroxide and chlorine The molar ratio of iridic acid is 36, and after the dropwise addition is completed, the stirring is continued for 2h under nitro...
Embodiment 2
[0036] A preparation method for an iridium-titanium composite catalyst, comprising the steps of:
[0037](1) Get 36mg of chloroiridic acid and add 120mL of pure water, ultrasonically prepare chloroiridic acid solution with iridium concentration of 0.1mg / mL for 5min, add sodium hydroxide solution with molar concentration of 0.1mol / L, adjust pH to about 6, then Add 0.23g of titanium powder with a particle size of 20-200nm, sonicate for 10min, then stir at 80°C for 2h to obtain a mixed solution;
[0038] (2) Nitrogen gas is passed into the mixed solution obtained in step (1) to remove dissolved oxygen, then it is placed in an ice-water bath, and a sodium hydroxide solution with a molar concentration of 1mol / L is added under stirring conditions, wherein the sodium hydroxide The molar ratio to chloroiridic acid is 1, and after the dropwise addition is completed, the stirring is continued for 0.5h under nitrogen to obtain a precursor solution;
[0039] (3) Place the precursor solut...
Embodiment 3
[0041] A preparation method for an iridium-titanium composite catalyst, comprising the steps of:
[0042] (1) Take 360 mg of chloroiridic acid and add 6 mL of pure water, ultrasonicate for 30 minutes to prepare a chloroiridic acid solution with an iridium concentration of 20 mg / mL, add a sodium hydroxide solution with a concentration of 0.01 mol / L, adjust the pH to about 6, and then add 280mg of titanium powder with a particle size of 20-200nm, ultrasonication for 10min, followed by stirring at 50°C for 0.5h to obtain a mixed solution;
[0043] (2) Nitrogen gas is passed through the mixed solution obtained in step (1) to remove dissolved oxygen, then it is placed in an ice-water bath, and a sodium hydroxide solution with a concentration of 1mol / L is added under stirring conditions, wherein sodium hydroxide and chlorine The molar ratio of iridic acid is 50, and after the dropwise addition is completed, the stirring is continued for 1h under nitrogen condition to obtain a prec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com