Probiotic mixing preparation with anti-influenza capability and application thereof
A probiotic and anti-influenza technology, applied in the field of microorganisms, can solve problems such as obvious side effects, and achieve the effect of great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0065] Example 1-1: Screening and identification of Lactobacillus mucosae
[0066] 1. Filter
[0067] Taking human feces as samples, the samples were pretreated and stored in -80°C refrigerator in about 20% glycerol. After taking out and thawing, mix the samples and add 0.5mL of the sample to 4.5mL to contain 0.05% cysteine. The 0.9% normal saline was used for gradient dilution, and the appropriate gradient dilution was applied to the MRS plate with 0.05% cysteine, and cultured at 37 °C for 48 hours. A single colony was transferred to liquid MRS medium (containing 0.05% cysteine) for enrichment, and stored in 30% glycerol to obtain strain CCFM1025 and strain F1.
[0068] 2. Identification
[0069] The genomes of CCFM1025 and F1 were extracted, and the 16S rDNA of CCFM1025 and F1 was amplified and sequenced (Shanghai Sangon Bioengineering Co., Ltd.), and the sequences were compared in GenBank. The results showed that the strain was Lactobacillus mucosae, named as Lactobacill...
Embodiment 1-2
[0070] Example 1-2: Culture of Lactobacillus mucosae
[0071] Lactobacillus mucosae CCFM1025 was inserted into MRS solid medium (containing 0.05% cysteine) and cultured at 37°C for 48 hours, and the colonies were observed and observed under the microscope. The colonies were found to be Round, rough, transparent, the cells are short rod-shaped.
[0072] Lactobacillus mucosae CCFM1025 was inserted into MRS medium (containing 0.05% cysteine) and cultured at 37°C for 48h, and a growth curve was made. It is a heterofermentation that produces acid and gas from glucose.
[0073] Lactobacillus mucosae CCFM1025 was inserted into MRS medium (containing 0.05% cysteine) and cultured at 10, 15, 20, 25, 30, 35, 40, 45, and 50 °C for 48 hours, respectively, and then observed. Growth conditions, good growth at 20-35 °C, still able to grow at 45 °C, but almost no growth at 15 °C or lower than 15 °C or 50 °C.
[0074] Lactobacillus mucosae CCFM1025 was inserted into MRS medium (containing 0....
Embodiment 1-3
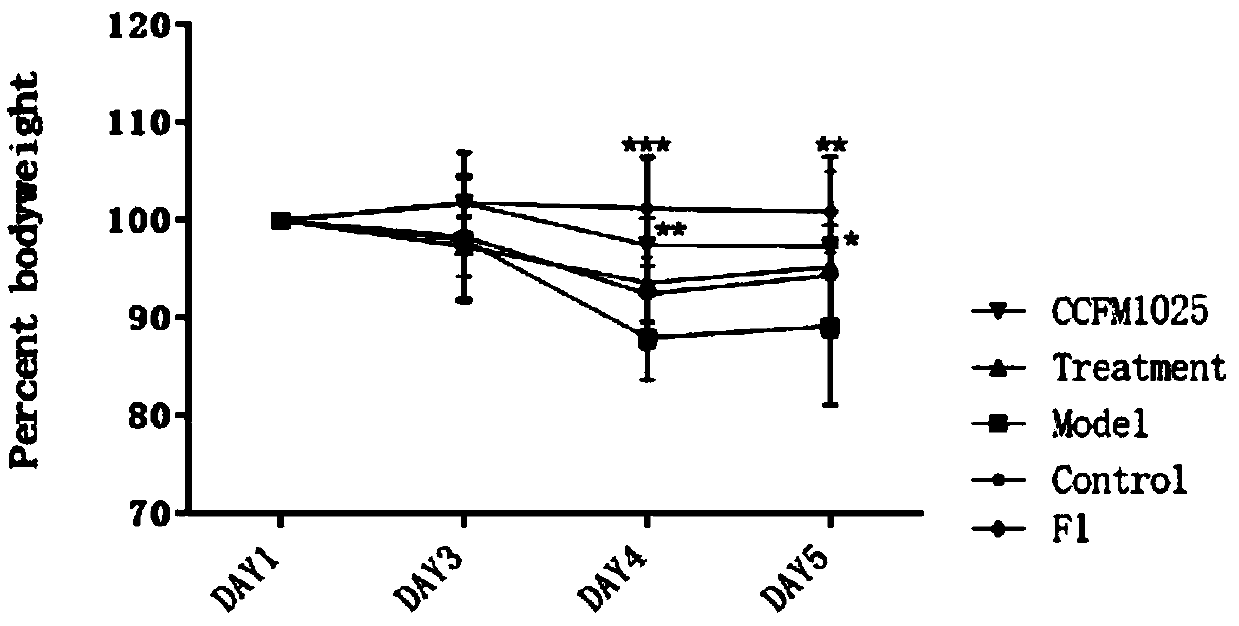
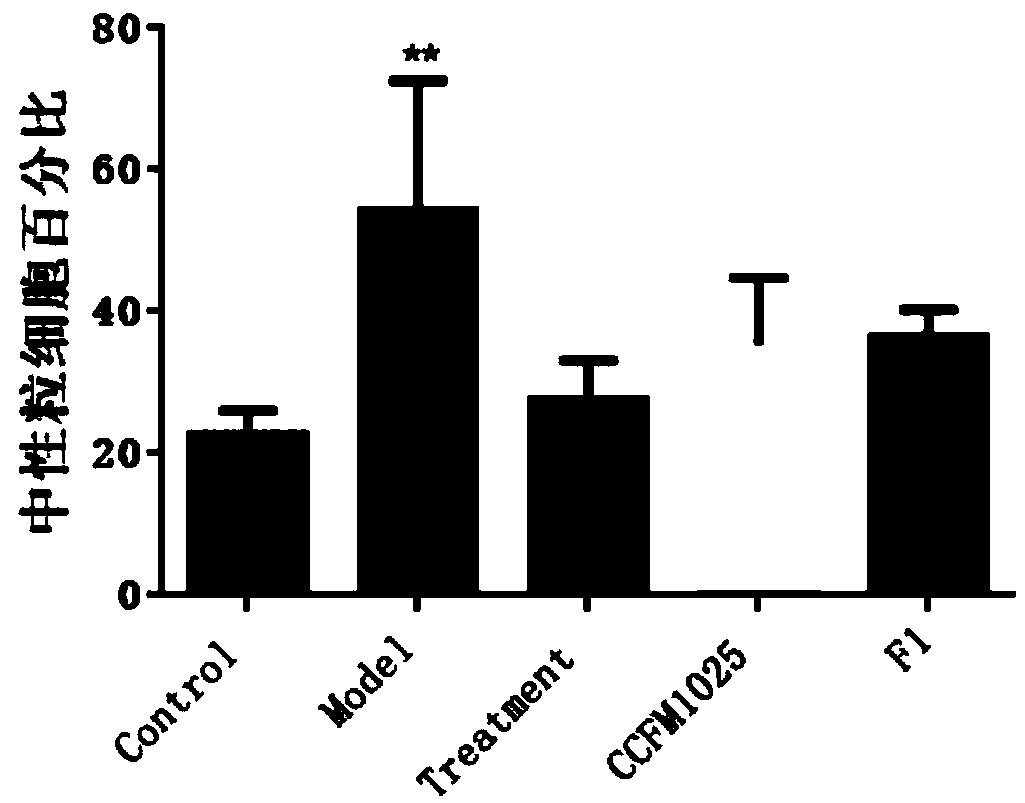
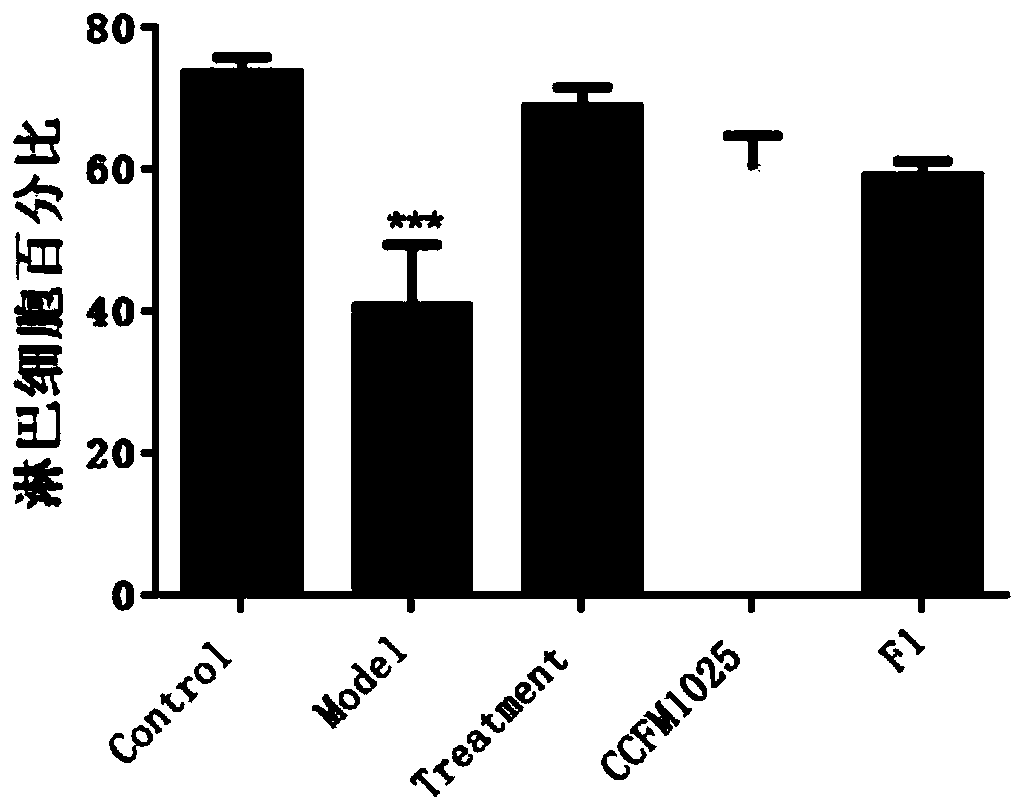
[0075] Example 1-3: Effects of Lactobacillus mucosae on the body weight of influenza mice
[0076] 40 healthy ICR female mice weighing 20-24g were taken and randomly divided into 5 groups. The 5 groups were named as blank control group (Control), influenza model group (Model), and ribavirin drug treatment group (Treatment). , Lactobacillus mucosae intervention group (CCFM1025) by gavage of Lactobacillus mucosae CCFM1025, Lactobacillus mucosae intervention group (F1) by gavage of Lactobacillus mucosae F1, 8 in each group.
[0077]Two weeks before the experiment, the Lactobacillus mucosae intervention group (CCFM1025) was given 10 gavage every day. 9 Lactobacillus mucosae CCFM1025 bacterial suspension dilution with the amount of CFU bacteria, Lactobacillus mucosae F1 intervention group (F1) was given the same amount of Lactobacillus mucosae F1 bacterial suspension dilution by gavage, and the other groups (Control, Model, Treatment) were given 0.2 mL per day. saline.
[0078] O...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com