Andrographolide nanometer suspension agent
A technology of andrographolide and nanosuspension, applied in the field of medicine, can solve the problems such as inability to realize continuous operation, limited scale-up production, and inconvenient process, achieve a small dispersion coefficient, avoid the growth of the particle size of the preparation, and simplify the process. controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Preparation of Andrographolide Nanosuspension—Comparison of Different Instruments
[0046] prescription:
[0047]
[0048] Process:
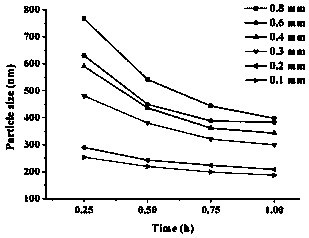
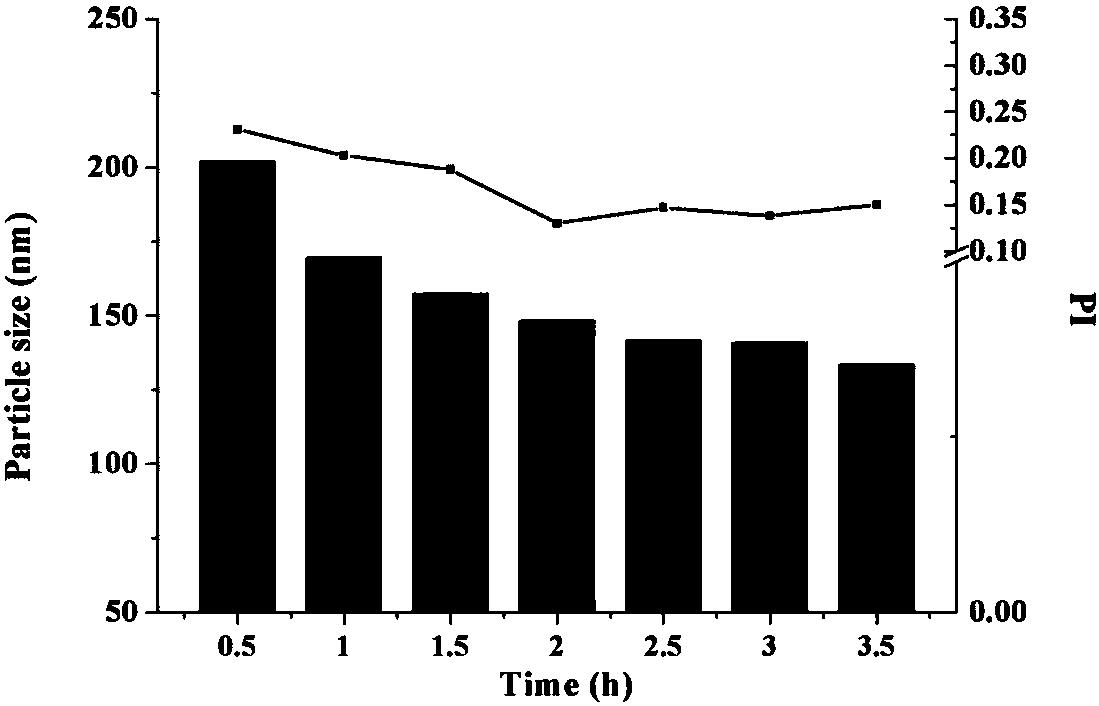
[0049] (1) ATS high-pressure homogenizer: Dissolve 0.2 g sodium deoxycholate in distilled water, add 6 g of andrographolide after complete dissolution, and use shear dispersion to completely disperse the drug in the aqueous solution containing stabilizers. The mixed solution was passed through a high-pressure homogenizer, 400 and 800 bar were circulated once, and then 1200 bar was circulated 20 times, and an appropriate amount of andrographolide suspension was taken out to measure the particle size to be 1.1 μm, which could not reach the nanometer level. Reduce the amount of prescription andrographolide to 0.6%, and use the same method to circulate through the high-pressure homogenizer 20 times, take out an appropriate amount of andrographolide suspension and measure the average particle size to be 532 nm.
[0050] (2) Micr...
Embodiment 2
[0054] Preparation of embodiment 2 andrographolide nanosuspensions——crude drug without micronization or through micronization
[0055] Take the unmicronized andrographolide raw material drug, adopt the same prescription as in Example 1 and the method in (4) in Example 1 to prepare andrographolide nanosuspensions respectively, and take out an appropriate amount of andrographolide in 0.5 h and 1 h before grinding. The particle size of the ester suspension was determined. Compared with Example 1 (4), that is, the nanosuspension prepared after the micronization of the crude drug. The results are shown in Table 1.
[0056] Table 1 Average particle size of nanosuspension prepared by micronization or non-micronization of andrographolide API
[0057]
[0058] It can be seen from Table 1 that the particle size of the API is smaller after micronization and grinding at the same time. But at the same time, the method of the present invention has a higher grinding efficiency for part...
Embodiment 3
[0059] The preparation of embodiment 3 andrographolide nanosuspensions—adding or not adding stabilizer
[0060] prescription:
[0061]
[0062] Process:
[0063] The process of (4) in Example 1 was used to prepare the andrographolide nanosuspension. Before grinding, 0.5, 1.0, and 1.5 h were taken out from the discharge port to measure the particle size of the andrographolide nanosuspension. Compared with the andrographolide nanosuspension prepared by adding stabilizer in Example 2. The results are shown in Table 2.
[0064] Table 2 The average particle size of andrographolide nanosuspension prepared with or without stabilizer in the formulation
[0065]
[0066] It can be seen from Table 2 that after adding a stabilizer, the particle size of the drug particles can be continuously reduced with the increase of grinding time, but without adding a stabilizer, the drug particles not only did not decrease but increased after grinding for 1 h. It can be seen that adding a s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com