Nutritional compositions with 2fl and lnnt for use in preventing and/or treating non-rotavirus diarrhea by acting on the gut microbiota dysbiosis
A technology of nutritional composition and microbiome, which can be applied to medical preparations containing active ingredients, drug combinations, and resistance to vector-borne diseases, etc., and can solve problems such as not considering the function of intestinal microorganisms.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0262] Table 1 below gives an example of the composition of a nutritional composition (eg infant formula) according to the present invention. This composition is given by way of example only.
[0263]
[0264]
[0265] Table 1 : Examples of the composition of nutritional compositions (eg infant formulas) according to the invention
Embodiment 2
[0267] Clinical Study Description
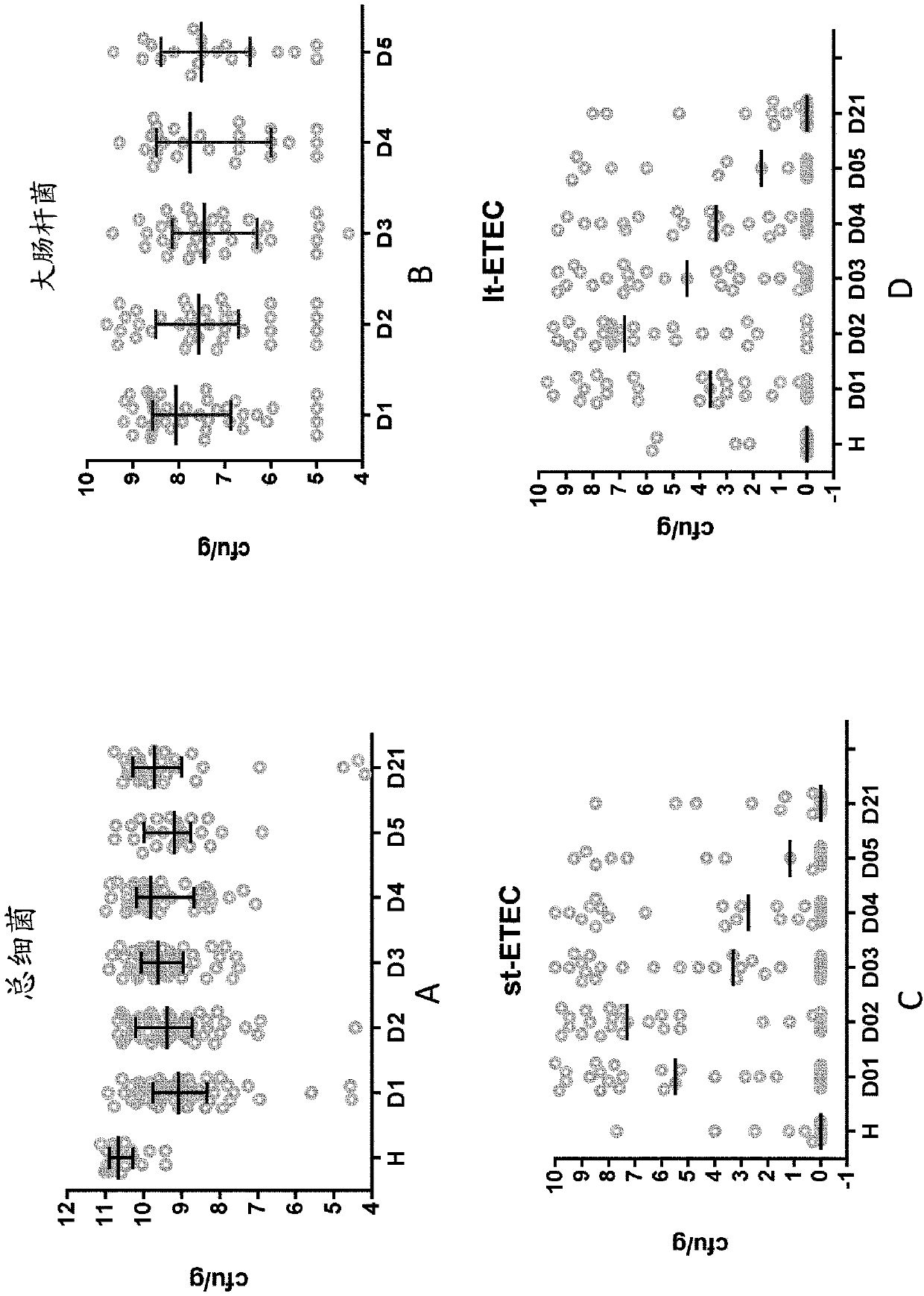
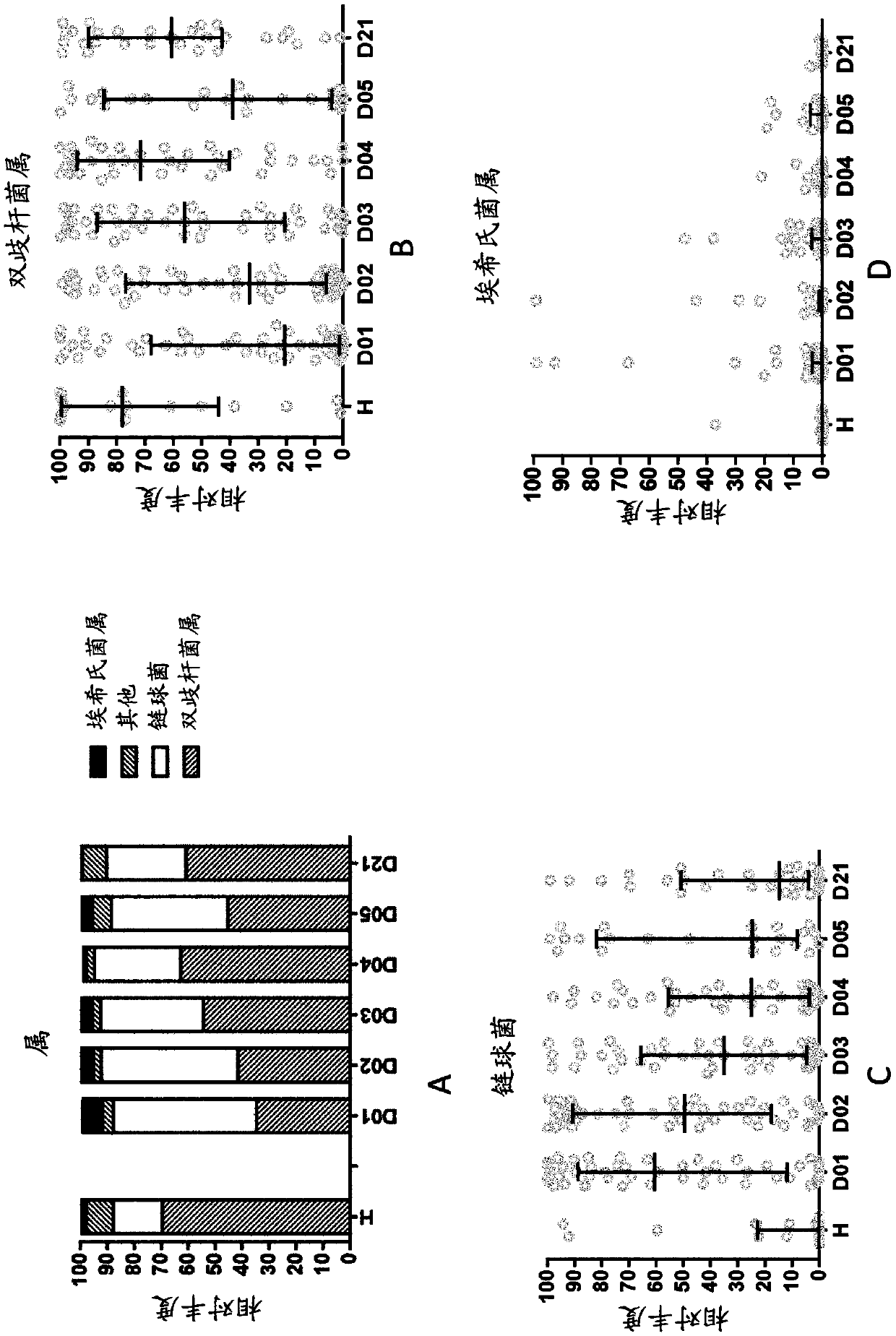
[0268] One hundred and twenty hospitalized infants and young children with acute diarrhea participated in this prospective single-centre study. Male (for easier separation of feces from urine) infants and young children aged 6 to 24 months with a history of watery diarrhea of less than 48 hours and who received written consent from their parents were included in the trial. Infants and young children with systemic infections, malnutrition (Z-score < -3), or major medical abnormalities were excluded. Infants and young children who had received or required antibiotics were also excluded from the trial. Infants and young children enrolled in the trial had to be negative for invasive diarrhea, negative for Vibrio cholerae on dark-field microscopy, and negative for rotavirus by ELISA in stool. Stool samples were investigated for the presence of ETEC, EPEC or EAEC according to standard procedures of icddr,b (Svenungsson B et al., Enteropathog...
Embodiment 3
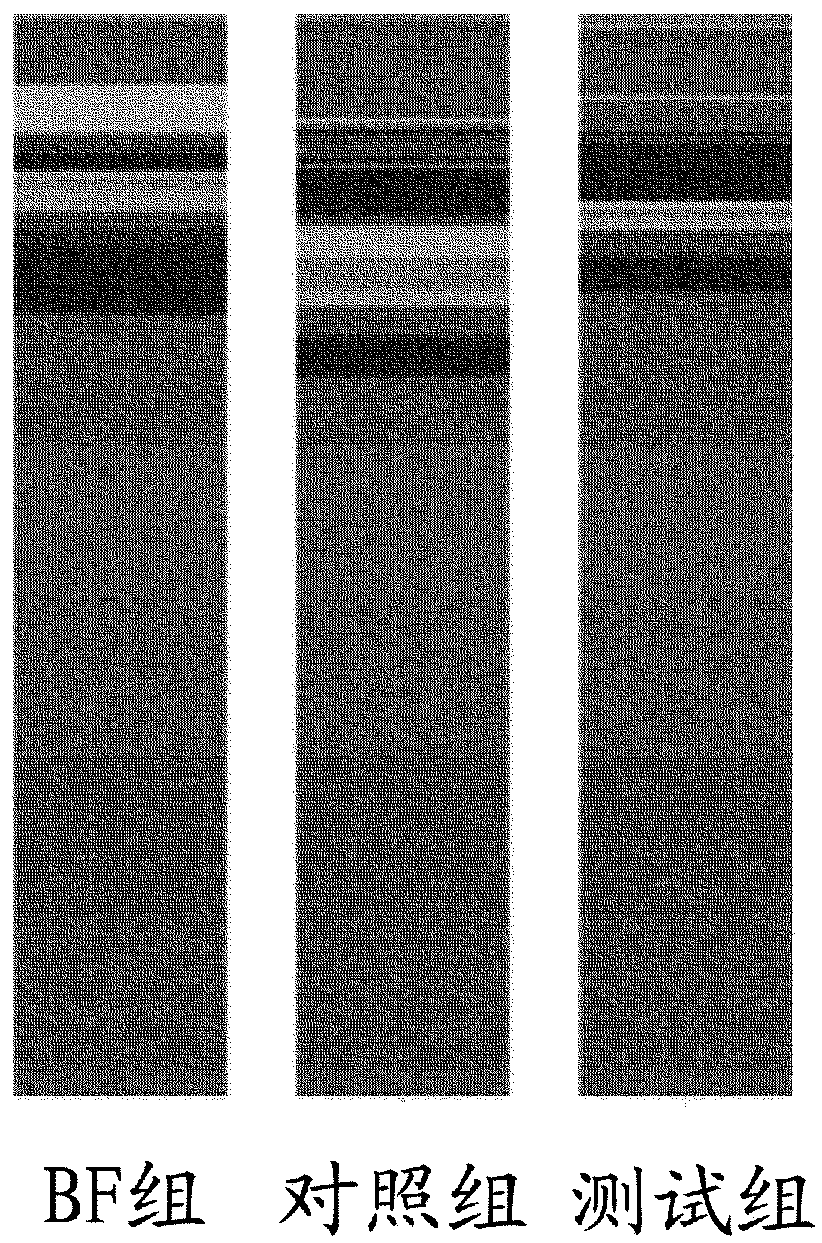
[0283] Clinical Study Description
[0284] Carried out at the Maternal and Fetal Unit, Neonatology Unit and Neonatal Intensive Care Unit (Dipartimento Materno Infantile, Unità Operativa Complessa di Neonatologia e Terapia Intensiva Neonatale) of AOUP "Paolo Giaccone" in Palermo, Italy and the Pediatric Unit (Kinderartsenpraktijk) in Hasselt, Belgium safety test.
[0285] This study is a randomized, controlled, two-center interventional clinical trial of 2 parallel formula-fed groups. The study population in the formula-fed group consisted of healthy full-term male and female infants, aged 0 to 14 days, who were exclusively formula-fed at enrollment. Eligible infants were randomly assigned to one of two study formulas (control or test), using method of delivery (vsc or cesarean) and sex as stratification factors. For Phase 1, randomized infants receive exclusive feeding of test or control formula from enrollment to 4 months of age in amounts appropriate for their body weigh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com