Kit for assisting in detection of placenta implantation and application thereof
An auxiliary detection and detection reagent technology, applied in the field of molecular biology, can solve the problems of no placenta accreta, high technical requirements, low specificity, etc., and achieve the effect of improving accuracy and fast detection means
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Materials and equipment
[0030] Kit: Bio-Plex Assay 171AC600M; instrument model: Bio-Plex 200; vortex mixer; refrigerated centrifuge suitable for 1.5-2ml centrifuge tubes; microplate shaker (up to 500rpm); magnetic separation plate ( Hand-Held Magnetic Plate Washer); 2μl-1000μl single-channel pipette; 20μl-300μl multi-channel pipette; multi-channel pipette reservoir; deionized water, beaker, test tube, absorbent paper, etc.
[0031] 2. Collection of peripheral blood samples
[0032] After obtaining consent, collect non-anticoagulated peripheral blood serum from suspected cases and 30-50 cases of normal delivery pregnant women before delivery, coagulate at room temperature for 30-45 minutes, and centrifuge at 1000g at 4°C for 15 minutes. minutes, and then transferred to cryopreservation tubes. Store in a -80°C ultra-low temperature freezer. After the samples were collected, 50 μl of serum was frozen and thawed at 4°C, and centrifuged repeatedly at 1000 g for 10 mi...
Embodiment 2
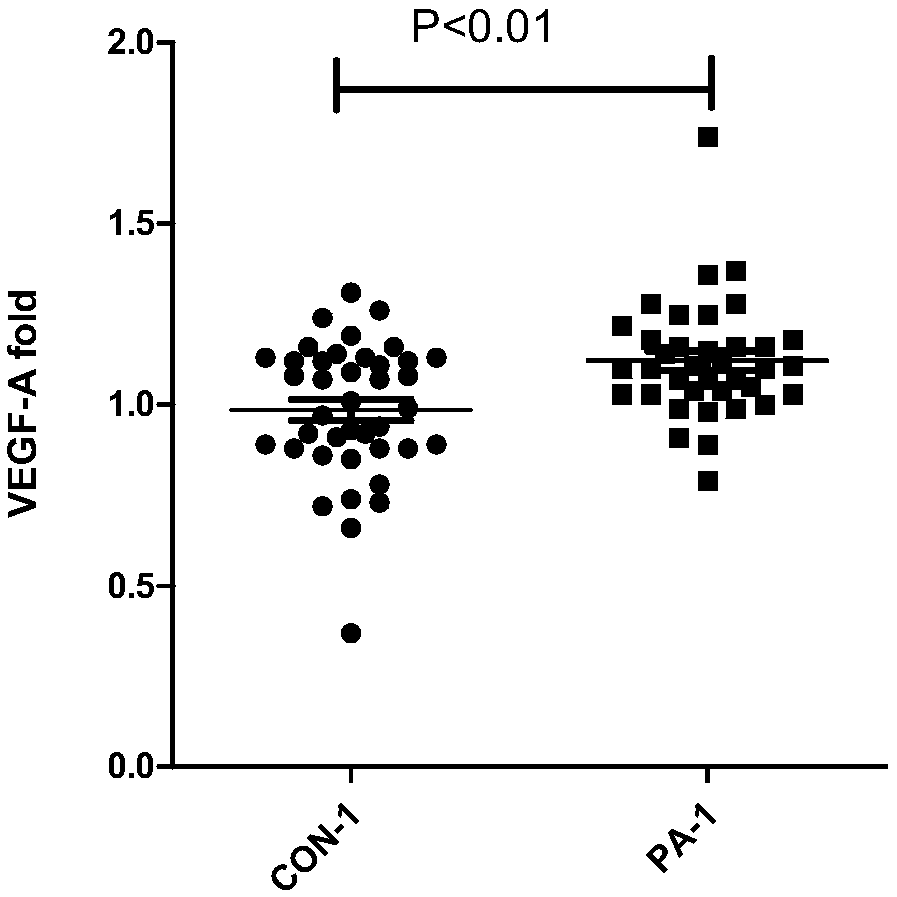
[0106] Using the same method as in Example 1, the non-anticoagulant peripheral blood serum collected before delivery was collected from pregnant women who were diagnosed as false positive by other methods before delivery, and from pregnant women who were pathologically diagnosed with placenta previa combined with placenta accreta. Perform VEGF-A concentration detection:
[0107] The first group of pregnant women with false positive prepartum diagnosis results were: pregnant women with placenta previa diagnosed by ultrasound but no placenta accreta occurred after delivery.
[0108] Test results such as Figure 7-8 shown, where Figure 7 P is the relative concentration of VEGF-A, P is the pregnant woman with placenta previa diagnosed by ultrasound but no placenta accreta, and PA-3 is the pregnant woman with placenta previa diagnosed by ultrasound and placenta accreta confirmed by pathology. Figure 8 is the ROC curve and its AUC value. The results showed that a cut-off value ...
Embodiment 3
[0113] According to the method described in Example 1, after obtaining consent, the VEGF-A concentration in the antepartum peripheral serum of 38 cases of normal delivery pregnant women from January 2017 to December 2017 was detected, and the average value was 423.0389. The average value is the reference normal value. Calculate the detection value / reference value ratio of 10 suspected cases from January 2017 to December 2017. The results are shown in Table 3:
[0114] Table 3 Application of VEGF-A detection in auxiliary diagnosis of placenta accreta
[0115]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com