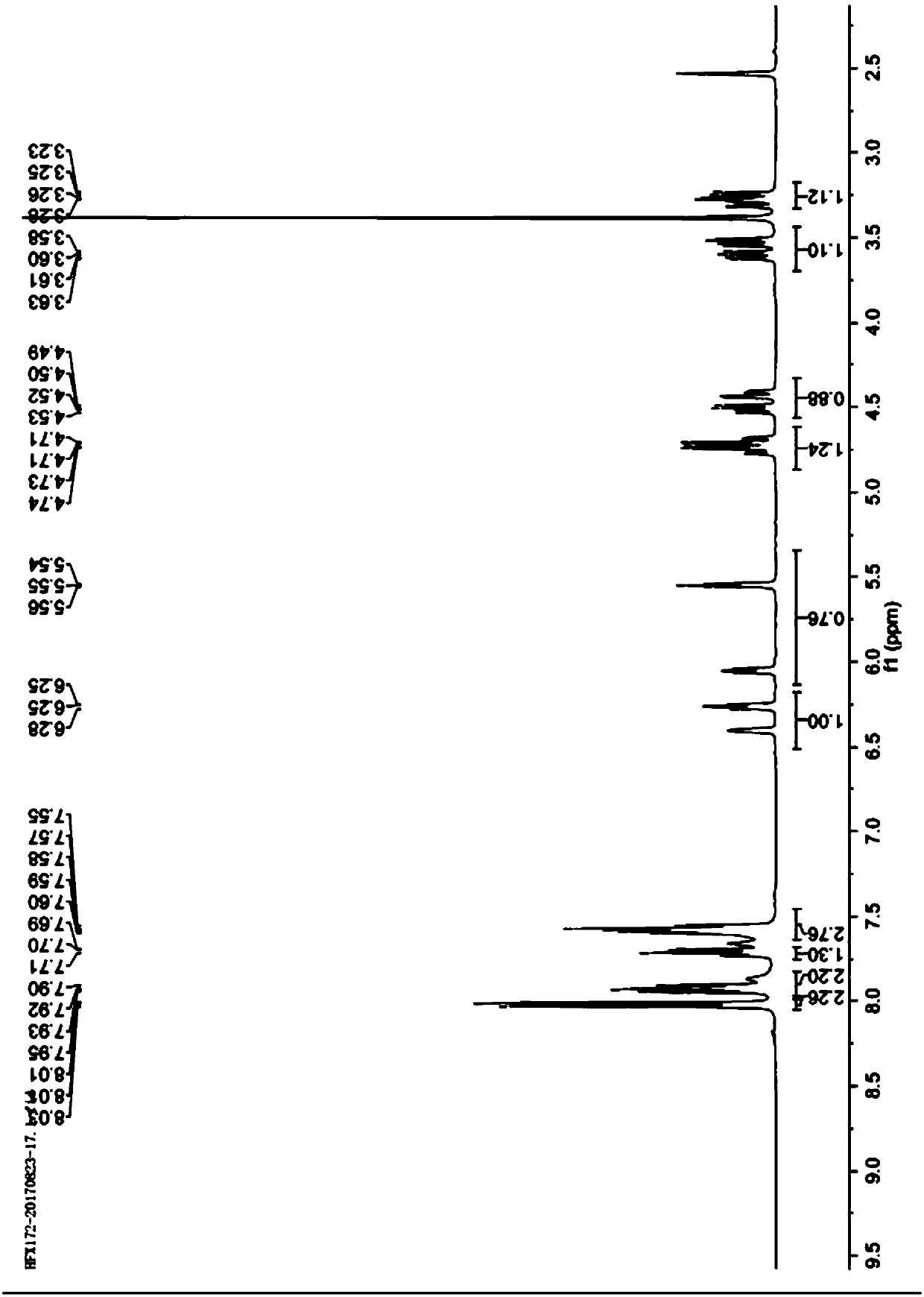
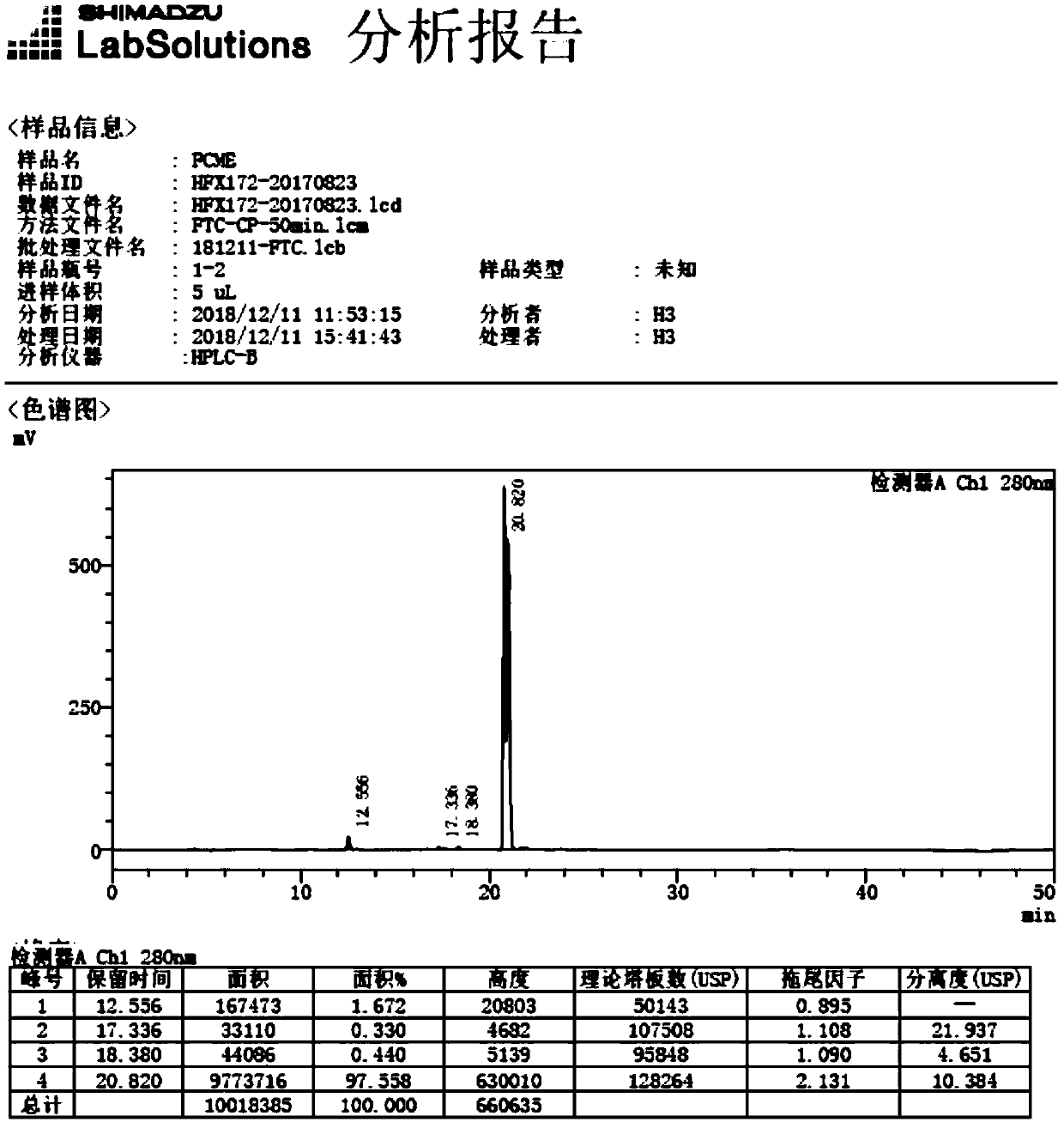
Preparation method for emtricitabine isomer
A technology of emtricitabine and isomers, which is applied in the field of drug synthesis, can solve the problems of incomplete synthesis, separation and separation of three isomers, etc., and achieve the effects of simple operation, easy purification operation, and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Preparation of Compound II (R is benzoyl): 54g (1.0eq) of acetonide was dissolved in a solution of 300ml of dichloromethane and 62g (1.5eq) of triethylamine, cooled to 0-5°C, and added dropwise within 1h Dissolve 60g (1.05eq) of benzoyl chloride solution in 60ml of dichloromethane, add dropwise and keep warm for about 1h. After the reaction was completed, 200 ml of water was added to the reaction mixture for liquid separation, and the organic layer was washed with 100 ml of water. The organic phase was evaporated to dryness and concentrated to dryness to obtain 90 g of oil, which was directly put into the next reaction without purification.
Embodiment 2
[0071] Preparation of Compound III: Dissolve 90 g of the oil obtained in the previous step in 200 ml of tetrahydrofuran, add 40 ml of 2N hydrochloric acid dropwise, heat to 45-50° C., stir for 2 hours, and then stir overnight at room temperature. After the reaction, neutralize with 5g of sodium bicarbonate and filter. The filtrate was evaporated to dryness to obtain 85 g of oil, which was directly put into the next reaction without purification.
Embodiment 3
[0073] Preparation of Compound IV: Dissolve 85g of the above oil in 400ml of dichloromethane and 40ml of water, and add 80g of sodium periodate (1.0eq) in 8 batches at 20-25°C. The reaction was continued for 4 hours. The solid was filtered, and the filtrate was concentrated to obtain 75 g of oil, which was directly put into the next reaction without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com