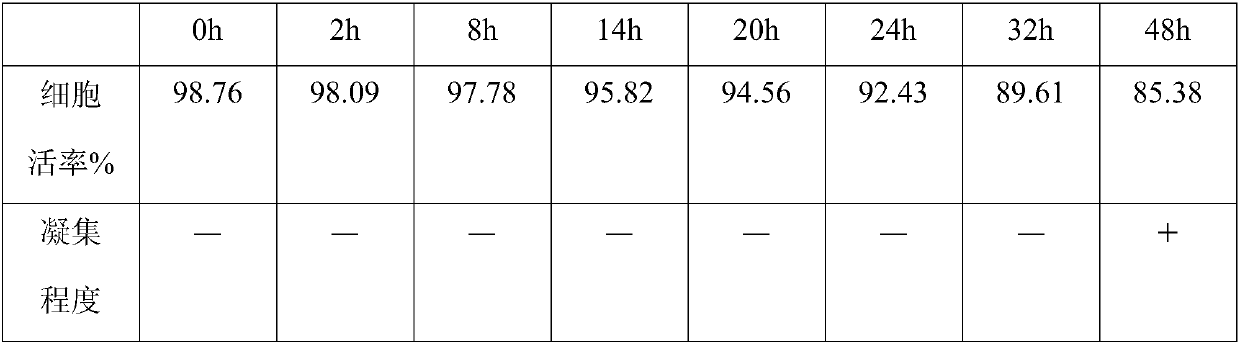
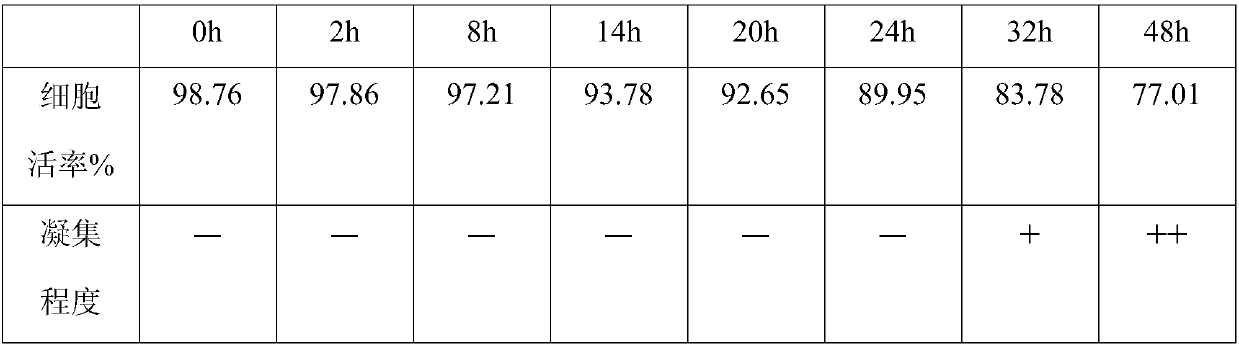
Mesenchymal stem cell preserving fluid for clinic local injection and method for preserving mesenchymal stem cell
A technology of stem cells and local injection, applied in the field of stem cell preservation solution, can solve the problems of low safety and short time to maintain cell viability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A mesenchymal stem cell preservation solution for clinical local injection, comprising the following components in volume percentage:
[0034] 3% autologous plasma lysate, 3% autologous stem cell conditioned medium extract, 1% antioxidant vitamin C injection, 0.4% hyaluronic acid, and the rest is physiological saline injection, all of which are clinical injection grade reagents.
[0035] Wherein, the autologous plasma lysate is prepared by the following method:
[0036] (1) Use 8 mL sodium citrate anticoagulant tubes to collect 16 mL of peripheral blood from the patient in 2 tubes;
[0037] (2) Put the collected peripheral blood into a centrifuge and centrifuge at a speed of 1500r / min for 10 minutes. After centrifugation, the peripheral blood is divided into four layers, which are plasma layer, buffy coat layer, and gel layer from bottom to top. Buffy coat layer and blood cell layer, absorb the plasma layer and gently blow the buffy coat layer, then mix the plasma and ...
Embodiment 2
[0044] A mesenchymal stem cell preservation solution for clinical local injection, comprising the following components in volume percentage:
[0045] 1% autologous plasma lysate, 1% autologous stem cell conditioned medium extract, 0.1% antioxidant vitamin C injection, 0.1% hyaluronic acid, and the balance is physiological saline injection.
[0046] The preparation method of autologous plasma lysate and autologous stem cell conditioned medium extract is the same as that in Example 1.
Embodiment 3
[0048] A mesenchymal stem cell preservation solution for clinical local injection, comprising the following components in volume percentage:
[0049] Autologous plasma lysate 5%, autologous stem cell conditioned medium extract 5%, antioxidant vitamin C injection 1%, hyaluronic acid 0.4%, and the balance is physiological saline injection.
[0050] The preparation method of autologous plasma lysate and autologous stem cell conditioned medium extract is the same as that in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com