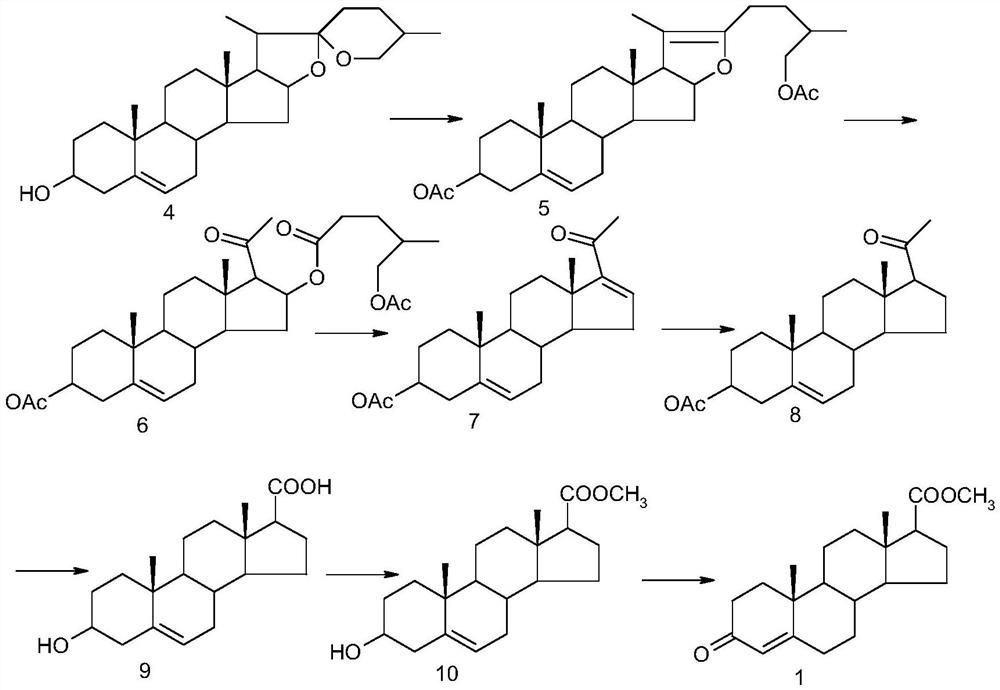
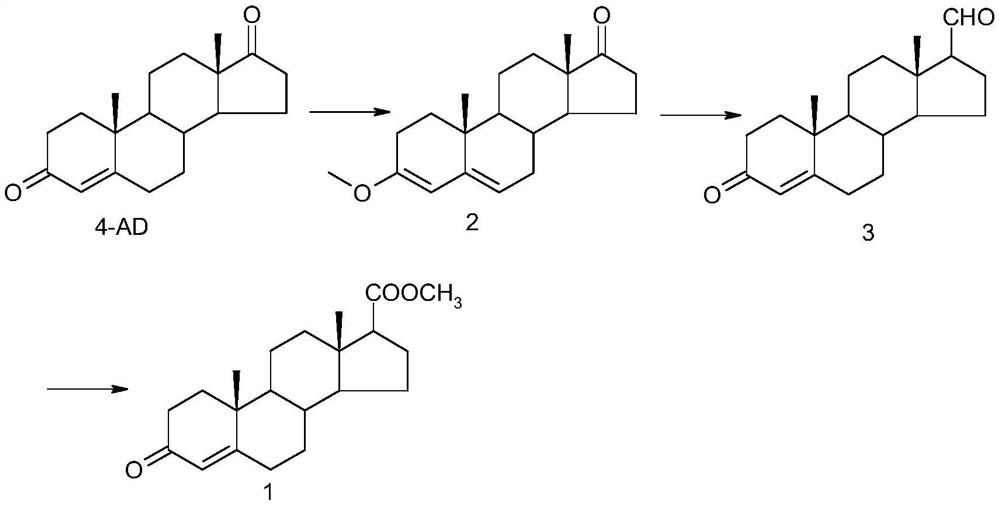
Synthetic method of androst-4-en-3-one-17β-carboxylate methyl ester
A technology of methyl carboxylate and a synthesis method, which is applied in the synthesis field of androst-4-ene-3-one-17β-carboxylate methyl ester, can solve the problems of high pollution, high cost, complicated process, etc. The effect of stable source, simple and simple process, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of 3-methoxy-androst-3,5-dien-17-one (2)
[0032] Under nitrogen protection, add 57.28 grams (0.2 moles) of 4AD, 250 milliliters of anhydrous tetrahydrofuran, 50 grams (0.471 moles) of trimethyl orthoformate, and 0.6 grams (3.15 millimoles) of p-toluenesulfonic acid to the reaction flask at 5 ° C Keep warm for 5 hours, and TLC detects the end point of the reaction. After the reaction, add 1 gram (10 mmol) of triethylamine, stir for 30 minutes, and terminate the reaction. Add 500 ml of water dropwise, stir and crystallize at 5°C for 2 hours, filter to obtain off-white crystals. After drying at 70°C, 58.77 g (0.195 mol) was obtained. Yield: 97.3%.
Embodiment 2
[0034] Synthesis of androst-4-en-3-one-17β-carbaldehyde (3)
[0035] Under the protection of nitrogen, 54.8 grams (0.16 moles) of (methoxymethyl) triphenylphosphorous chloride were added to the reaction flask, 500 milliliters of anhydrous tetrahydrofuran, 18.5 grams (0.165 moles) of potassium tert-butoxide were added, and stirred at room temperature for 1 Hour. Add 30.14 g (0.1 mol) of 3-methoxy-androst-3,5-dien-17-one, and react at room temperature for 12 hours. The end point of the reaction was detected by TLC. After the reaction was complete, 150 ml of 6N hydrochloric acid solution was added dropwise, and stirred at room temperature for 3 hours. The end point of the reaction was detected by TLC. After the reaction was complete, sodium bicarbonate solution was added dropwise to adjust the pH to 7, tetrahydrofuran was recovered under reduced pressure, the temperature was lowered to 5°C, and kept for 2 hours, filtered, and vacuum-dried at 60°C to obtain androst-4-en-3-one -1...
Embodiment 3
[0037] Synthesis of androst-4-en-3-one-17β-methyl carboxylate (1)
[0038] Add 30 g (0.1 mol) of androst-4-en-3-one-17β-formaldehyde into the reaction flask, 300 ml of anhydrous methanol, and 1.66 g (10 mmol) of potassium iodide, control the temperature at 20°C, and add dropwise 27 grams (0.3 moles) of oxidized tert-butanol was added in about 30 minutes, and the temperature was raised to reflux for 6 hours. TLC detected that the reaction was complete, and 27.7 grams (0.22 moles) of sodium sulfite (dissolved in 100 milliliters of water) was added to terminate the reaction, and concentrated under reduced pressure. Add 200 ml of water, stir at room temperature for 2 hours, filter, and dry at 60°C to obtain 30.75 g (0.093 mol) of off-white solid, yield: 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com