A mutant of cyclodextrin glucosyltransferase and its application
A glucose-based, mutant technology, applied in the fields of genetic engineering and enzyme engineering, to achieve the effect of improving the conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Expression of wild-type cyclodextrin glucosyltransferase
[0032] Cgt / pET20b(+) / BL21(DE3) (Yang Yulu, Wang Lei, Chen Sheng, etc.) was inoculated from glycerol tubes stored in the laboratory in the early stage. The production process of β-cyclodextrin glucosyltransferase Condition optimization [J]. Biotechnology Bulletin, 2014, 8:175-181.) Grow in LB liquid medium (containing 100mg / L ampicillin) for 8h, insert the seeds into TB liquid fermentation medium ( Contains 100mg / L ampicillin). After Escherichia coli was cultured and fermented on a shaking table at 25°C for 48 hours, a certain volume of fermentation broth was centrifuged at 4°C and 12,000 rpm for 15 minutes, and the fermentation supernatant was taken, which was the fermented crude enzyme liquid.
Embodiment 2
[0033] Example 2: Preparation and expression of cyclodextrin glucosyltransferase single mutant
[0034] (1) Preparation of mutants
[0035]According to the gene sequence of Bacillus circulans cyclodextrin glucosyltransferase, primers for introducing M234I, M234A, M234L, and M234V mutations were designed and synthesized, and the cyclodextrin glucosyltransferase gene Cgt was subjected to site-directed mutation, and the cyclodextrins were confirmed by sequencing. Whether the coding gene of the glucosyltransferase mutant is correct; the vector carrying the mutant gene is introduced into Escherichia coli for expression, and a single mutant cyclodextrin glucosyltransferase is obtained.
[0036] PCR amplification of the gene encoding the site-directed mutant: using the rapid PCR technique, the expression vector Cgt / pET-20b(+) carrying the gene encoding wild-type cyclodextrin glucosyltransferase was used as a template.
[0037] The site-directed mutagenesis primers for introducing th...
Embodiment 3
[0062] Example 3: Analysis of dismutation activity of cyclodextrin glucosyltransferase and its affinity for maltose receptor
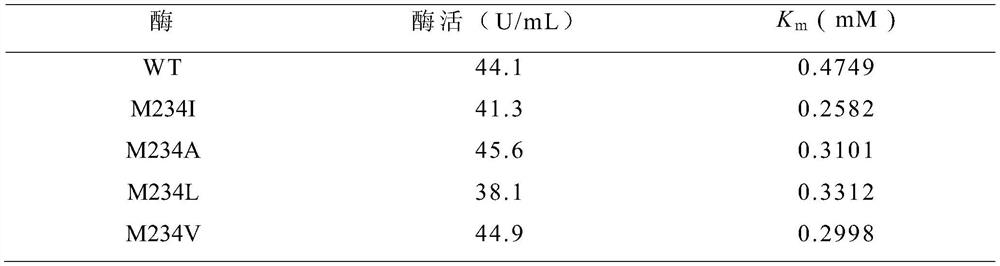
[0063] The fermented supernatant crude enzyme liquid obtained in Example 1 and Example 2 was subjected to enzyme activity determination. Enzyme activity and kinetic parameter K of wild-type cyclodextrin glucosyltransferase (WT) and mutants m Listed in Table 1, the results showed that all mutants had higher receptor affinity to maltose than the wild type.
[0064] Table 1 Shake Flask Enzyme Dismutation Activity of Wild-type and Mutant Cyclodextrin Glucosyltransferase and Its Affinity with Maltose as Acceptor
[0065]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com