Organic photoelectric material containing thiophene structure and application thereof
A technology of organic optoelectronic materials and thiophene, which is applied in luminescent materials, organic chemistry, circuits, etc., can solve the problems of low luminous efficiency and short service life of devices, and achieve the advantages of avoiding compact accumulation, high thermal stability, and high-efficiency electroluminescent performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
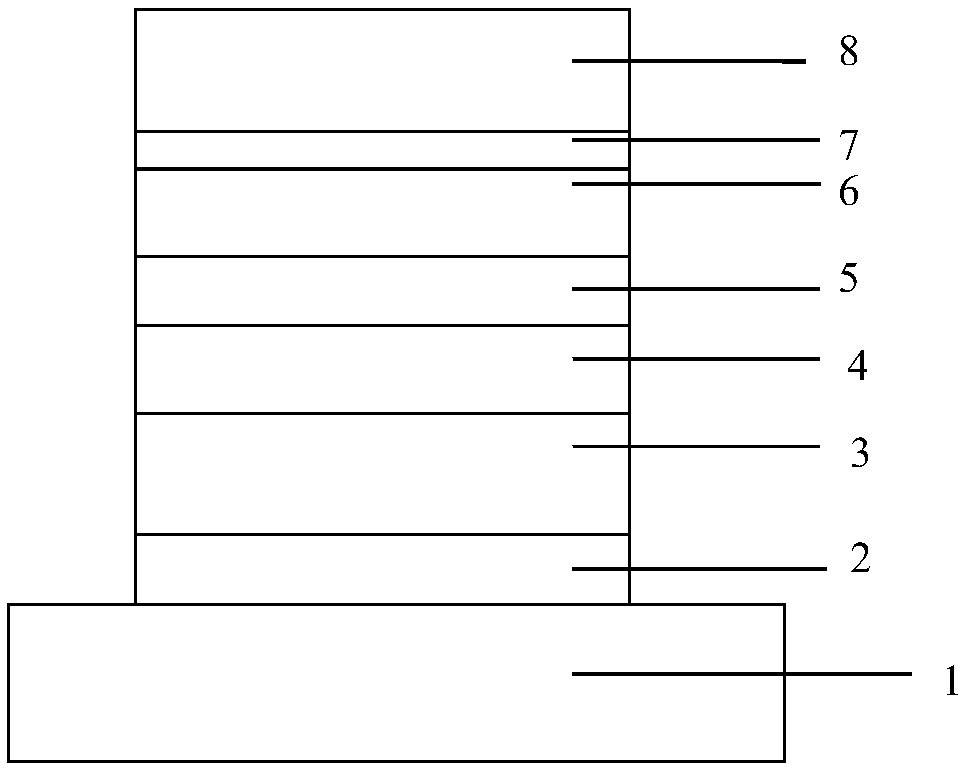
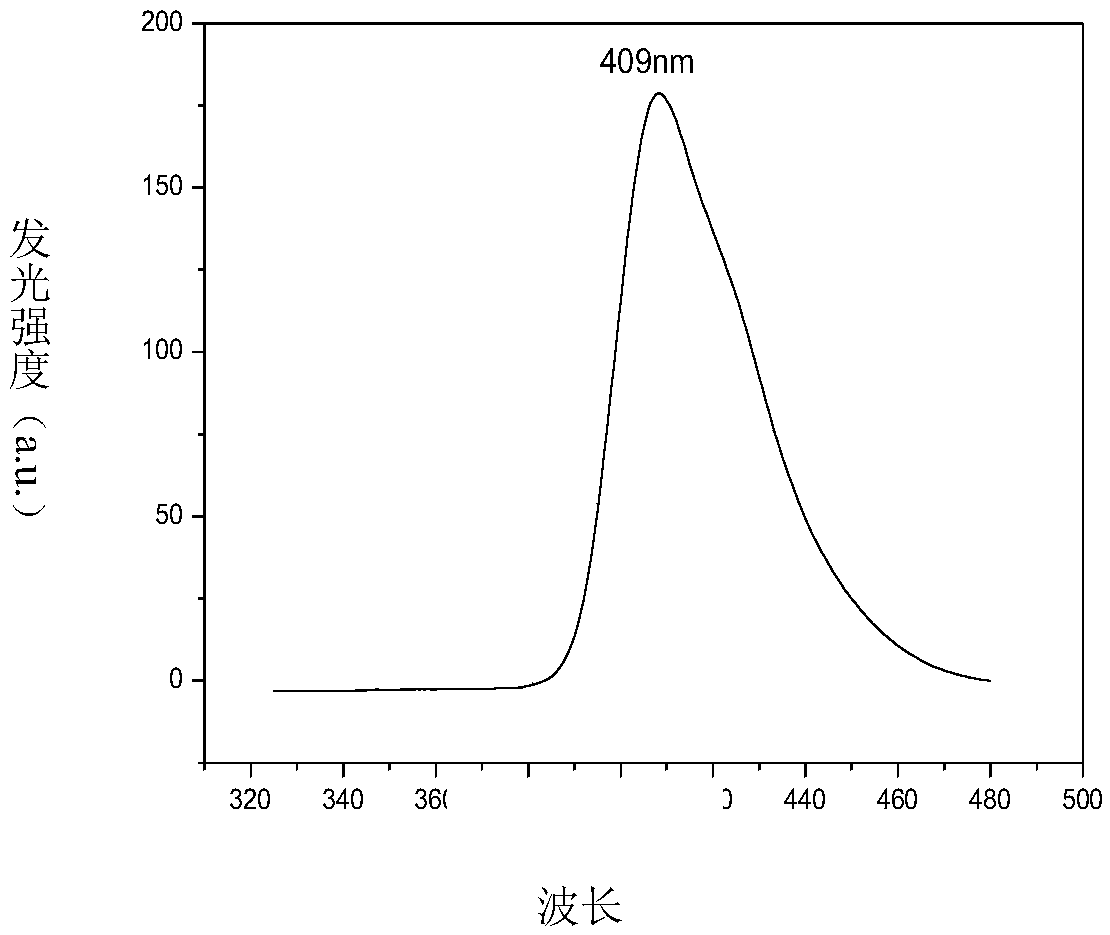
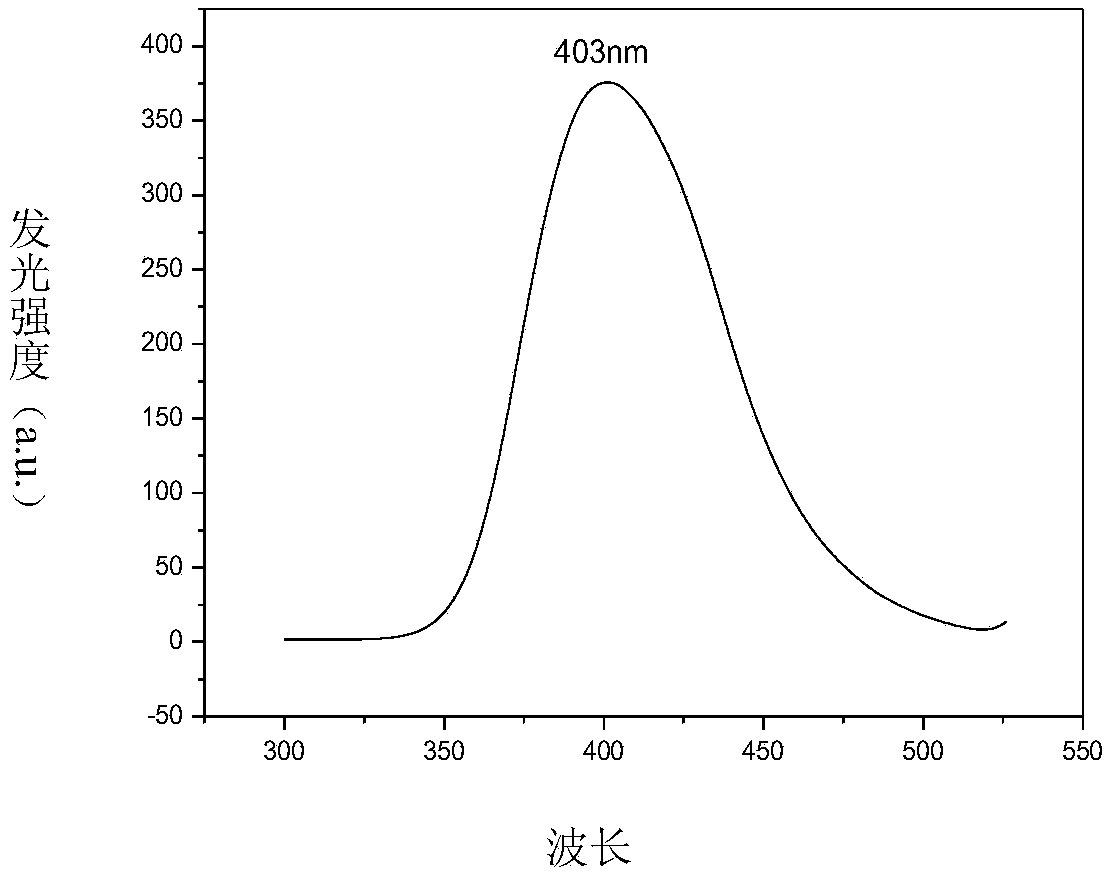
Image
Examples
Embodiment 1
[0042] The synthesis of embodiment 1 compound 1:
[0043] Add 6.4g (22.0mmol) (4-(9H-carbazol-9-yl) phenyl) boric acid and 4.0g 2-(2,6-dibromo-4H-cyclopenta[1,2 -b: 5,4-b']dithiophen-4-yl)malononitrile (10.0mmol), add 60mL toluene, then add 30.0mL K 2 CO 3 aqueous solution (1 mol / L), and stirred for 1 hour under nitrogen gas to remove oxygen in the reaction flask. Then 12 mg (0.1 mmol) of palladium tetrakistriphenylphosphine was added, and refluxed under vigorous stirring, and the reaction process was tracked and detected by TLC. After reacting for 24 hours, add 50 mL of deionized water to the reaction solution, filter to remove the insoluble matter, separate the water phase from the organic phase, and concentrate the organic phase to no fraction by distillation under reduced pressure. Esters: n-Hexane (1:10, v / v). 5.63 g of white solid were obtained (77.88% yield). Using DEI-MS to identify the compound, formula C 48 h 26 N 4 S 2 , detection value [M+1] + =723.63, calc...
Embodiment 2
[0044] The synthesis of embodiment 2 compound 2
[0045] Using (4-(9,9-dimethylacridin-10(9H)-yl)phenyl)boronic acid as the starting material, it was prepared according to the synthetic method of compound 1 in Example 1. Using DEI-MS to identify the compound, formula C 54 h 38 N 4 S 2 , detection value [M+1] + =807.75, calculated value 806.25.
Embodiment 3
[0046] The synthesis of embodiment 3 compound 3
[0047] Using (4-(10H-phenoxazin-10-yl)phenyl)boronic acid as the starting material, it was prepared according to the synthetic method of compound 1 in Example 1. Using DEI-MS to identify the compound, formula C 48 h 2 6N 4 o 2 S 2 , detection value [M+1] + =755.09, calculated value 754.15.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com