Immobilized xylanase, preparation method and application of immobilized xylanase
A technology for xylanase and xylo-oligosaccharide, which is applied in the fields of genetic engineering and enzyme engineering, can solve the problems of difficult acquisition of immobilization materials, complicated immobilization process, high price, etc., and achieves excellent immobilization effect, easy acquisition, and high cost. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] This example illustrates the method for preparing the immobilized support and extracting xylan.
[0024] Pretreatment of corncobs: crush corncobs (with a hemicellulose content of 30.5%), pass through a 20-40 mesh sieve, and take the under-sieve as crushed corncobs. Add distilled water ten times its mass to the crushed corncobs, place in a boiling water bath for 30 minutes, stir continuously during this period, centrifuge after cooling, discard the supernatant, rinse the precipitate with distilled water three times repeatedly, and dry.
[0025] Weigh 10 g of the pretreated corn cob into a 500 mL Erlenmeyer flask, add 200 mL, 40 g / L NaOH aqueous solution, stir at room temperature for 12 h, and obtain the filtrate and solid residue after suction filtration. The solid residue was washed with distilled water until neutral, and dried to obtain the corncob residue after alkali treatment, that is, the immobilized carrier. The pH of the filtrate was adjusted to 5.0-6.0 with 6M ...
Embodiment 2
[0027] This example illustrates the preparation method of expansin-xylanase fusion protein.
[0028] The amino acid sequence of the expansin-xylanase fusion protein is shown in SEQ ID NO: 1, and the fusion protein is named EXCL-EK2-XYN. Expansin-xylanase fusion protein (abbreviated as recombinant fusion protein) was prepared by engineering bacteria BL21(DE3) carrying the EXCL-EK2-XYN coding gene (see the invention patent application with application number 201710134735.7 and publication number CN108570107A for the construction method) ). The specific method is as follows: Pick a single colony of engineered bacteria from a plate cultivated overnight at 37°C, inoculate it into a 50mL Erlenmeyer flask containing 10mL of LB liquid medium, and cultivate it at 37°C until the logarithmic growth phase. Take 0.5 mL of the recombinant bacterial liquid and inoculate it into a 250 mL Erlenmeyer flask filled with 50 mL of LB liquid medium (containing 100 μg / mL of ampicillin kanamycin), an...
Embodiment 3
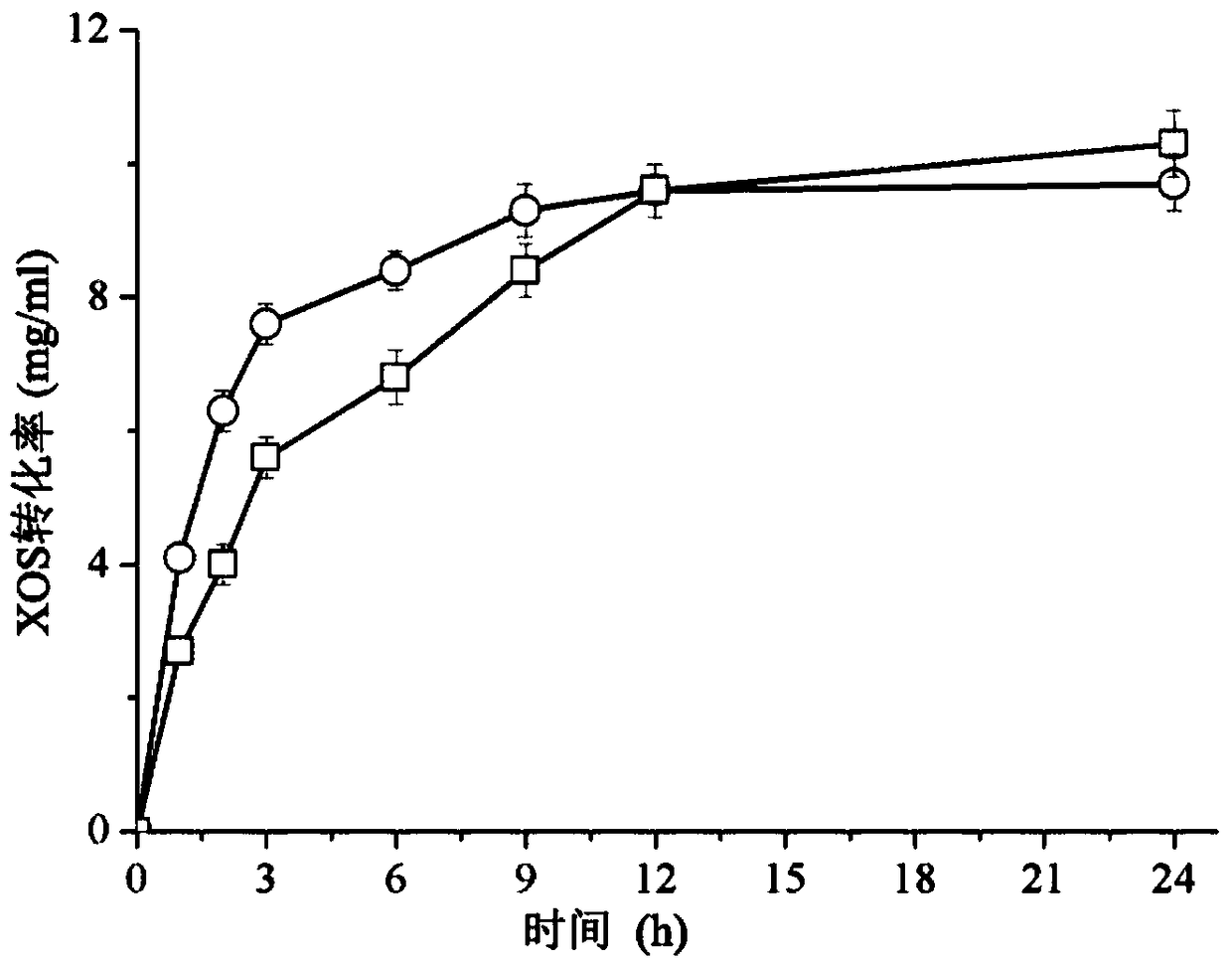
[0031] This example illustrates a method for highly efficient immobilization of recombinant fusion proteins.
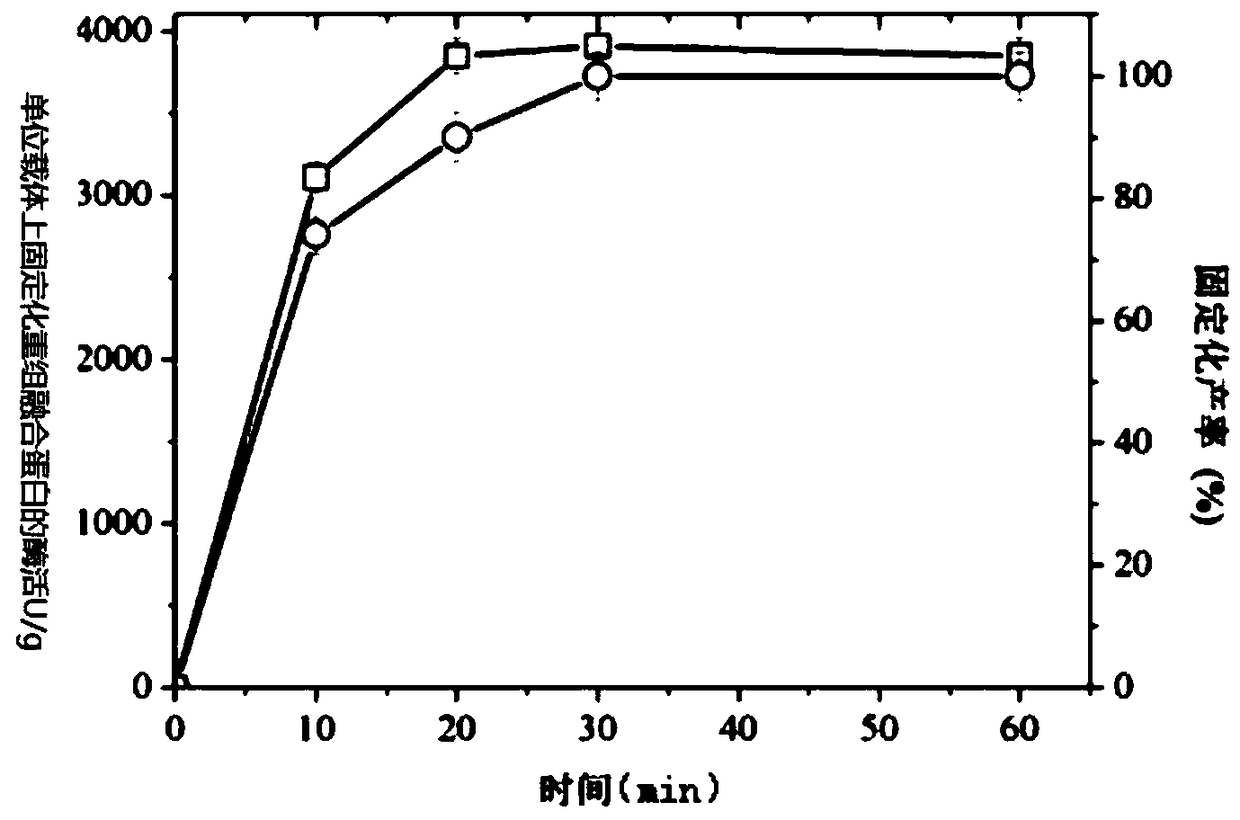
[0032] The immobilized carrier (prepared in Example 1) was added to the recombinant fusion protein crude enzyme solution (232 U / mL) prepared in Example 2, and 50 g of the immobilized carrier was added to each liter of the crude enzyme solution. The mixture of the immobilized carrier and the crude enzyme solution was placed in a shaker at 20° C. and 150 rpm for adsorption reaction. After the adsorption reaction, the precipitate was collected by centrifugation, washed three times with pH 7.0 phosphate buffer, and the immobilized recombinant fusion protein was obtained. After the adsorption reaction time was 10min, 20min, 30min, 40min, 50min, and 60min, the immobilized yield of recombinant fusion protein, the recovery rate of immobilized recombinant fusion protein and the specific activity of xylanase of immobilized recombinant fusion protein were investigated.
[0033]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com