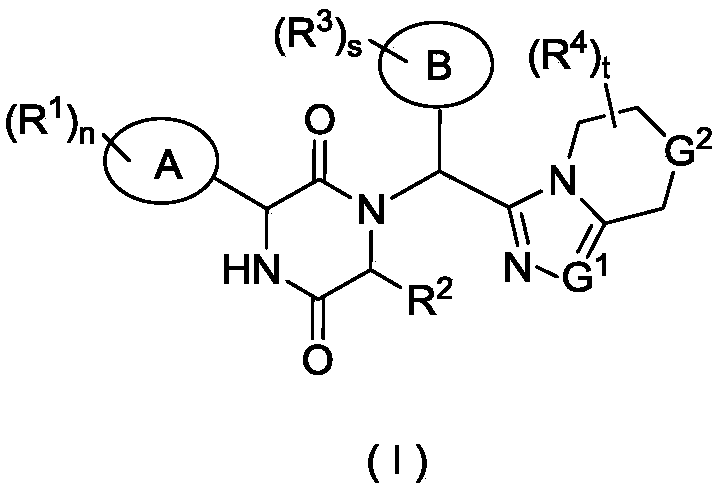
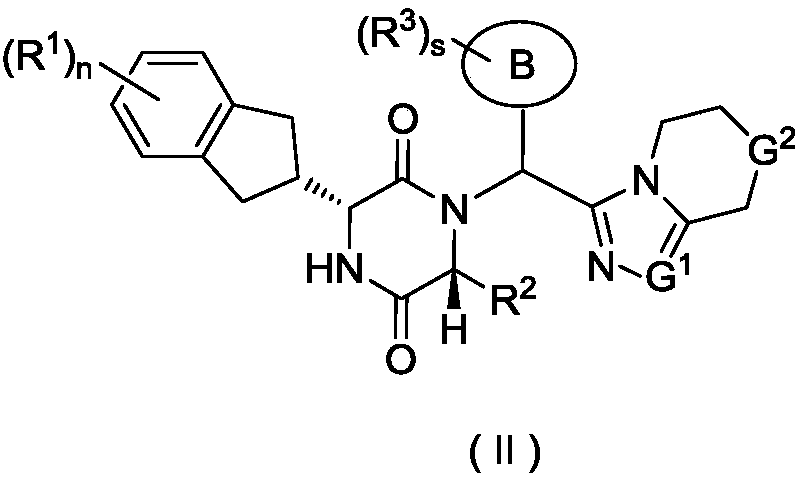
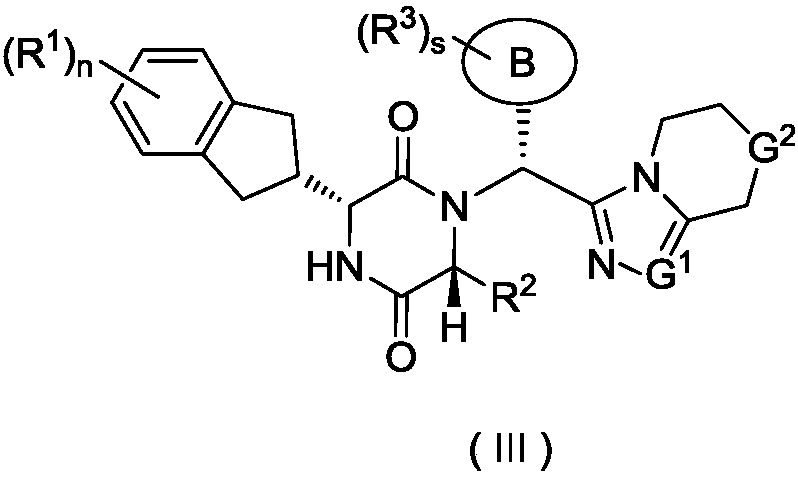
Piperazine-2,5-dione derivative, as well as preparation method and application thereof to medicine
A compound and mixture technology, applied in the field of medicine, can solve problems such as low oral bioavailability, toxic side effects, and respiratory arrest
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0211] Compound structures were determined by nuclear magnetic resonance (NMR) or / and mass spectroscopy (MS). NMR shift (δ) in 10 -6 (ppm) is given. The mensuration of NMR is to use Bruker AVANCE-400 nuclear magnetic instrument, and measuring solvent is deuterated dimethyl sulfoxide (DMSO-d 6 ), deuterated chloroform (CDCl 3 ), deuterated methanol (CD 3 OD), the internal standard is tetramethylsilane (TMS).
[0212] MS was measured using a FINNIGAN LCQAd (ESI) mass spectrometer (manufacturer: Thermo, model: Finnigan LCQadvantage MAX).
[0213] High performance liquid chromatography (HPLC) was analyzed using Agilent HPLC 1200DAD, Agilent HPLC1200VWD and Waters HPLC e2695-2489 high pressure liquid chromatography.
[0214] Chiral HPLC analysis and determination use column CHIRALPAK IE (150*4.6mm, 5μm (with guard column).
[0215] The thin-layer chromatography silica gel plate uses Yantai Huanghai HSGF254 or Qingdao GF254 silica gel plate. The specification of the silica gel...
Embodiment 9
[0321] (3R,6R)-6-((S)-sec-butyl)-3-(2,3-dihydro-1H-inden-2-yl)-1-((6,8-dihydro-5H- Imidazo[5,1-c][1,4]oxazin-3-yl)(2-methyloxazol-4-yl)methyl)piperazine-2,5-dione 9
[0322]
[0323]
[0324] first step
[0325] (3R,6R)-6-((S)-sec-butyl)-3-(2,3-dihydro-1H-inden-2-yl)-1-(2-(3-(hydroxymethyl) Morpholine)-1-(2-methyloxazol-4-yl)-2-oxoethyl)piperazine-2,5-dione 9b
[0326] Dissolve compound 1a (380 mg, 0.74 mmol) in 5 mL of 1,4-dioxane, add N, N-carbonyldiimidazole (143.13 mg, 0.88 mmol), react at room temperature for 1 hour, add morpholin-3-yl Methanol 9a (112.03mg, 0.96mmol) was reacted at 85°C for 2 hours. After the reaction, it was concentrated under reduced pressure, and the resulting residue was purified by thin-layer chromatography with developer system A to obtain the title compound 9b (180 mg, 46.6%).
[0327] second step
[0328] 4-(2-((2R,5R)-2-((S)-sec-butyl)-5-(2,3-dihydro-1H-inden-2-yl)-3,6-dioxo Piperazin-1-yl)-2-(2-methyloxazol-4-yl)acetyl)morpholine-3-...
Embodiment 10
[0337] (3R,6R)-6-((S)-sec-butyl)-3-(2,3-dihydro-1H-inden-2-yl)-1-((R)-(6,8-di Hydrogen-5H-imidazo[5,1-c][1,4]oxazin-3-yl)(2-methyloxazol-4-yl)methyl)piperazine-2,5-dione 10
[0338]
[0339] Compound 9 (82 mg, 0.163 mmol) was purified by HPLC (Waters 2767-SQ Detecor2, eluent system: water, acetonitrile, 0.05% trifluoroacetic acid) to obtain the title compound 10 (22 mg).
[0340] MS m / z (ESI): 504.5 [M+1].
[0341] 1 H NMR (400MHz, CDCl 3 )δ7.82(s,1H),7.25-7.19(m,4H),6.87(s,1H),6.26(s,1H),4.91-4.82(m,2H),4.11-3.99(m,5H) ,3.97-3.79(m,2H),3.29-3.07(m,4H),2.80-2.77(m,1H),2.44(s,3H),1.26(s,1H),0.95-0.87(m,4H) ,0.67-0.63(m,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com