Method for efficient expression preparation of UDP-glucose-hexose-1-phosphate uridyltransferase
A high-efficiency expression technology of uridine phosphate, applied in the direction of transferase, microbial-based methods, biochemical equipment and methods, etc., can solve the problems of unsuitable for large-scale preparation, low protein expression, cumbersome and time-consuming operations, etc. problem, to achieve the effect of low cost, simple and fast cultivation, and high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
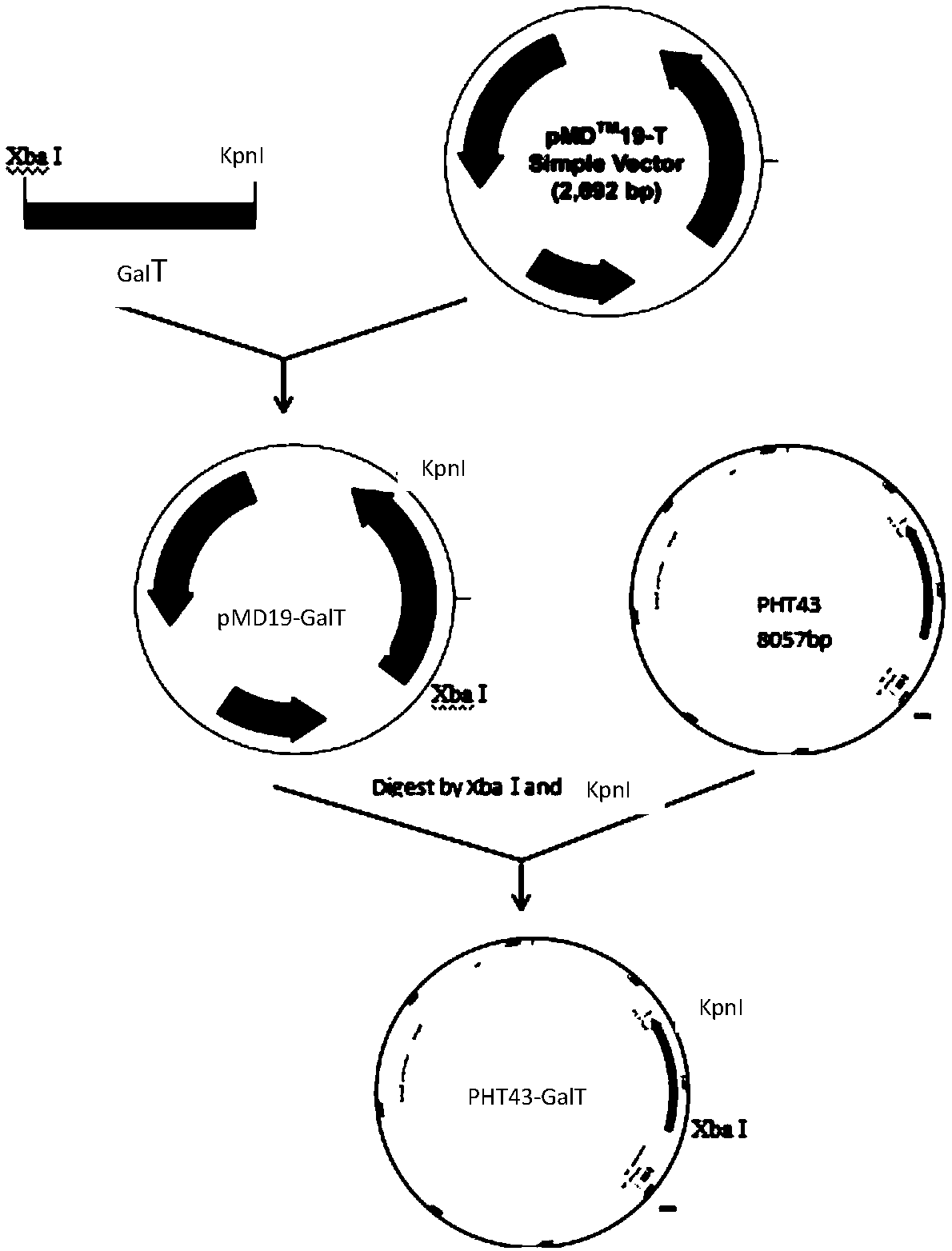
[0023] (1) Construction of recombinant expression vector pHT43-GalT
[0024] UDP-glucose-hexose-1-phosphate uridine acyltransferase (GalT) gene (sequence shown in SEQ ID NO: 1) is derived from Bifidobacterium longum JCM 1217, and after PCR amplification and purification, it is connected to the cloning vector pMD19, The recombinant plasmid pMD19-GalT was constructed.
[0025] The recombinant plasmid pMD19-GalT and the expression vector pHT43 were double-digested with XbaI and KpnI respectively, and ligated overnight at 16°C to obtain the recombinant expression vector pHT43-GalT.
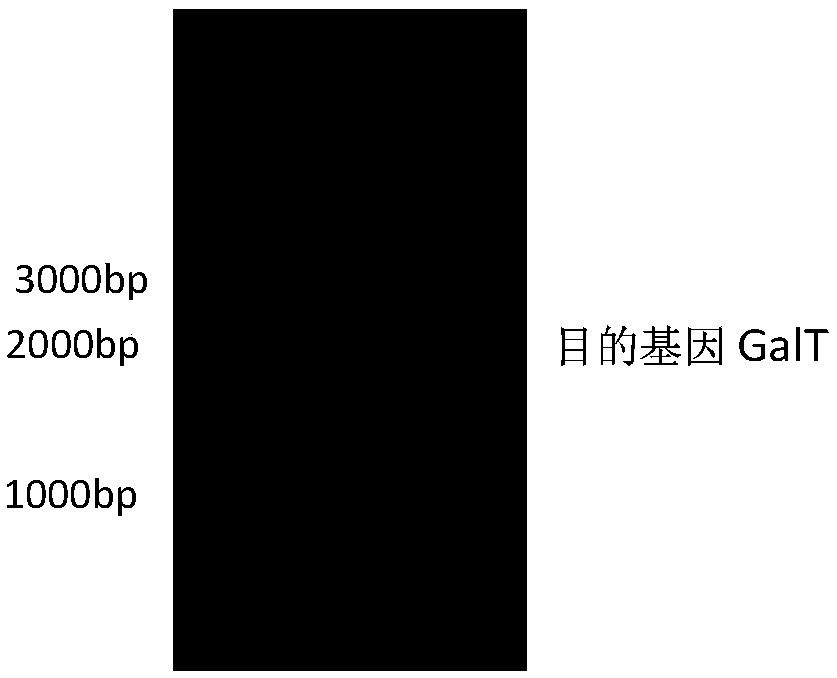
[0026] The recombinant expression vector pHT43-GalT was transformed into Bacillus subtilis WB800N, spread on LB plates containing chloramphenicol (5ug / mL) resistance, cultured overnight at 37°C, picked transformants, extracted recombinant plasmids and verified by double enzyme digestion. like figure 2 As shown, there are two fragments after digestion, the sizes are about 8000bp (expression vector p...
Embodiment 2
[0043] (1) Construction of recombinant expression vector pHT43-GalT
[0044] The recombinant plasmid pMD19-GalT prepared in Example 1 and the expression vector pHT43 were double-enzyme digested with XbaI and KpnI as the restriction sites respectively, and ligated at 16°C overnight to obtain the recombinant expression vector pHT43-GalT.
[0045] The recombinant expression vector pHT43-GalT was transformed into Bacillus subtilis WB800, coated with LB plate containing chloramphenicol (5ug / mL) resistance, cultured at 37°C overnight, the transformants were picked, the recombinant plasmid was extracted and verified by double digestion. like figure 2 As shown, there are two fragments after enzyme digestion, the sizes are about 8000bp (expression vector pHT43) and 2637bp (UDP-glucose-hexose-1-phosphate uridyltransferase), indicating that the connection is successful.
[0046] (2) Recombinant engineering bacteria
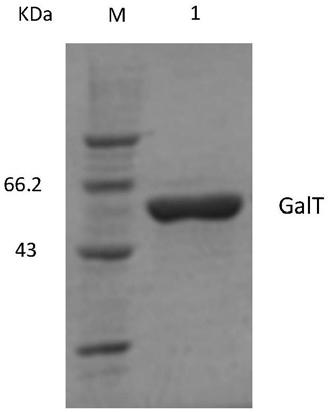
[0047] The constructed recombinant expression vector pHT43-GalT was ...
Embodiment 3
[0053] (1) Construction of recombinant expression vector pMA5-GalT
[0054] The recombinant plasmid pMD19-GalT prepared in Example 1 and the shuttle vector pMA5 were double digested with XbaI and KpnI as the restriction sites respectively, and ligated at 16°C overnight to obtain the recombinant expression vector pMA5-GalT.
[0055] The recombinant expression vector pMA5-GalT was transformed into Bacillus subtilis 168, coated with an LB plate containing ampicillin (100ug / mL) resistance, cultured at 37°C overnight, the transformants were picked, the recombinant plasmid was extracted and double-enzyme digestion was verified. Build succeeded.
[0056] (2) Recombinant engineering bacteria
[0057] The constructed recombinant expression vector pMA5-GalT was transformed by electric shock. Bacillus subtilis 168 electrotransformed competent cells were mixed with the recombinant expression vector pMA5-GalT plasmid, added to the electric shock cup in an ice bath for 5 minutes, and elect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com