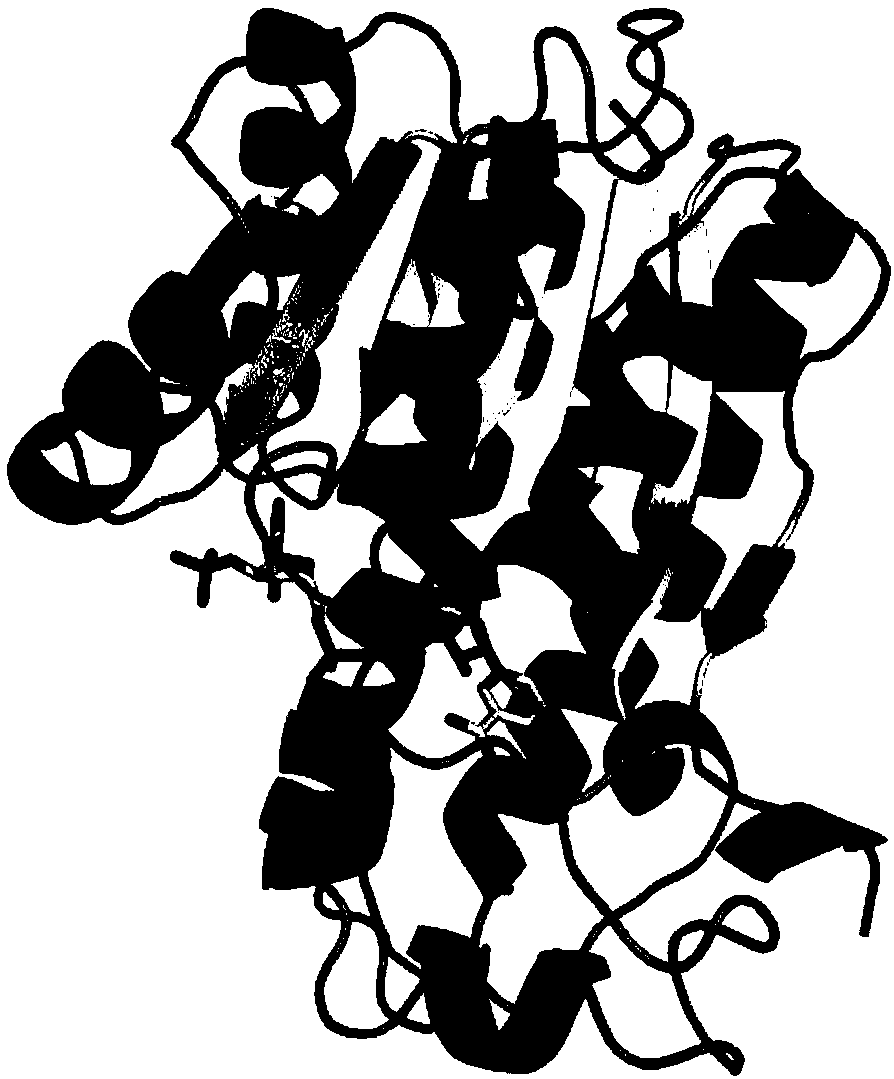Alcohol dehydrogenase mutant and use thereof
A technology of alcohol dehydrogenase and mutants, which is applied in the field of enzyme engineering and can solve the problems of large mutant library and dependence on high-throughput screening methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] 1. Semi-rational design of alcohol dehydrogenase LkADH
[0074] 1.1 Site-directed mutations at positions 147 and 202
[0075] (1) F147L, F147I, F147M, F147C, A202L, A202I, A202V mutants
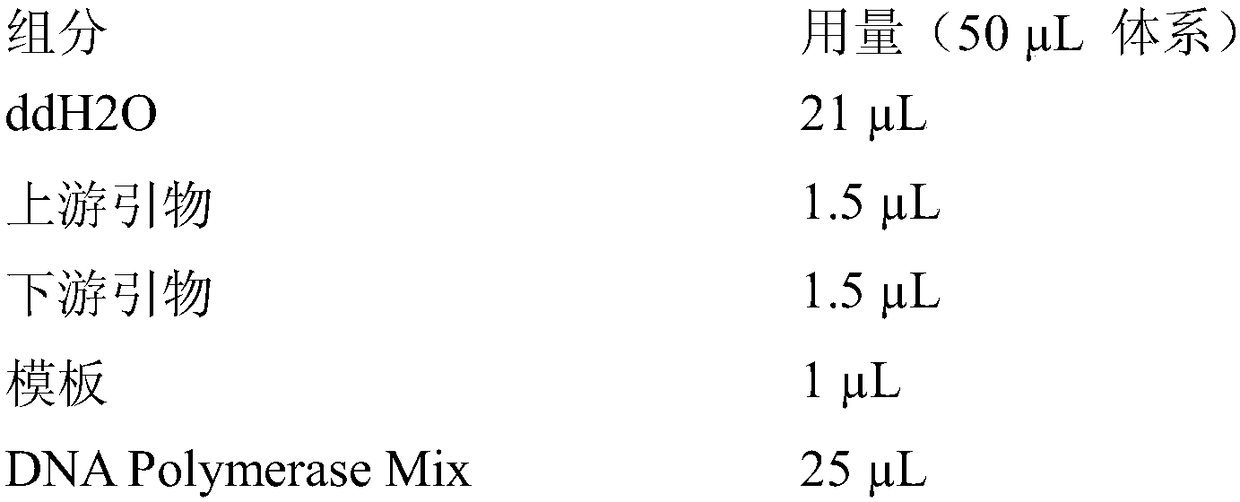
[0076] Using the recombinant plasmid Pet-30a(+)-LkADH connected with the alcohol dehydrogenase LkADH gene as a template, a pair of forward and reverse primers F-Primer / R-Primer (Table 1, 2) containing mutation sites were designed. The 147th and 202nd positions were subjected to site-directed mutagenesis by PCR.
[0077] Table 1 147-site site-directed mutagenesis primer sequence
[0078]
[0079]
[0080] Table 2 202 site-directed mutagenesis primer sequences
[0081]
[0082] (2) Obtaining F147L / A202L, F147I / A202L mutants:
[0083] A pair of forward and reverse primers F-Primer / R-Primer (Table 3), performing site-directed mutagenesis by PCR.
[0084] Table 3 147, 202 combination mutation primer sequence
[0085]
[0086] As shown in Table 4, site-directed mutations w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com