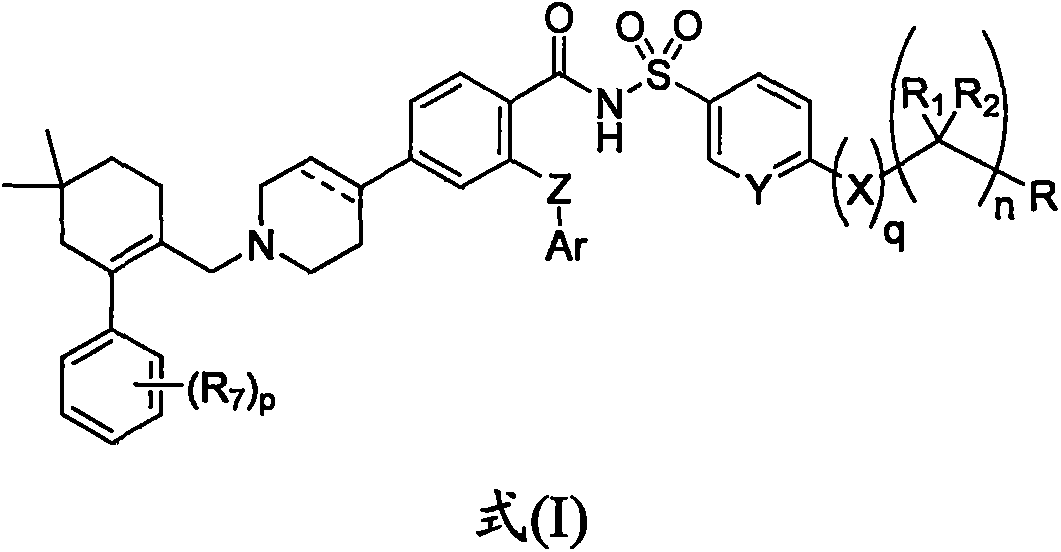
Bcl-2 selective inhibitor, and preparation method and usage thereof
A technology selected from, CH2, applied in the direction of organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Step A: 5-Bromo-1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]pyridine
[0121]
[0122] 5-Bromo-1H-pyrrolo[2,3-b]pyridine (3.94g) was dissolved in anhydrous THF (60mL), cooled to 0°C, and 1N LiHMDS solution in THF (2.2mL) was added, after 10 minutes, Triisopropylchlorosilane (4.24 g) was added, slowly raised to room temperature, and stirred for 40 hours. The reaction solution was poured into water, extracted with ethyl acetate, the organic phase was washed with saturated aqueous sodium chloride, dried over anhydrous sodium sulfate, and the solvent was removed to obtain the product (7.0 g).
[0123] 1 H NMR (400MHz, CDCl 3)δ8.27(d, J=2.4Hz, 1H), 7.98(d, J=2.4Hz, 1H), 7.31(d, J=3.6Hz, 1H), 6.49(d, J=3.6Hz, 1H) , 1.78~1.89 (m, 3H), 1.12 (d, J=7.6Hz, 18H).
[0124] Step B: Dimethyl (1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)boronate
[0125]
[0126] Under nitrogen protection, 5-bromo-1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]pyridine (5.37g) was dissolved in an...
Embodiment 2
[0190] Step A: 1-Morpholinyl-2-(2-nitrophenyl)ethyl-1-one
[0191]
[0192] 2-(2-Nitrophenyl)acetic acid (9 g) was suspended in dichloromethane (100 mL), cooled to 0°C. N,N-Dimethylformamide (0.5 mL) was added, and oxalyl chloride (6.35 mL) was slowly added dropwise. After the dropwise addition was completed, return to room temperature and stir for 2 hours. It was then concentrated in vacuo to obtain an oil, which was dissolved in dichloromethane (20 mL) and dropped into a 0° C. dichloromethane solution (100 mL) containing morpholine (5.2 mL) and triethylamine (17 mL). After the dropwise addition, return to room temperature and react overnight. The reaction solution was poured into water, extracted with ethyl acetate, the organic phase was washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, and the solvent was removed to obtain a crude product. The product (10.7g) was obtained after washing with petroleum ether / dichloromethane.
[0193] ...
Embodiment 3
[0209] Step A: 6-Chloropyridyl-3-methanesulfonyl chloride
[0210]
[0211] 6-Chloropyridinyl-3-amine (1.77 g) was dissolved in concentrated hydrochloric acid (14 mL), and cooled to -5°C. Slowly add an aqueous solution (12 mL) of sodium nitrite (1.04 g) dropwise. During the dropwise addition, keep the temperature between -5°C and 0°C. After the dropwise addition, keep it below 0°C for later use.
[0212] Slowly add thionyl chloride (4.5mL) dropwise to water cooled to -3°C, keep the temperature below 7°C, return to room temperature after dropwise addition, add cuprous chloride (70mg), cool to -5°C ℃, add the previously prepared diazonium salt solution, stir for 30 minutes and then filter. The filter cake was dissolved in ethyl acetate, dried over anhydrous sodium sulfate, and the solvent was removed to obtain the product (1.1g).
[0213] 1 H NMR (400MHz, CDCl 3 ) δ9.04 (d, J = 2.4 Hz, 1H), 8.25 ~ 8.28 (m, 1H), 7.61 (d, J = 8.4 Hz, 1H).
[0214] Step B: 6-Chloropyridyl-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com