Medicinal composition combining paclitaxel with novel phthalazinone compound
A compound, the technology of phthalazinone, which is applied in the field of the combined pharmaceutical composition of paclitaxel and novel phthalazinone compounds, can solve the problem of ineffectiveness of anticancer agents, and achieve the effect of improving biological activity and anticancer curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
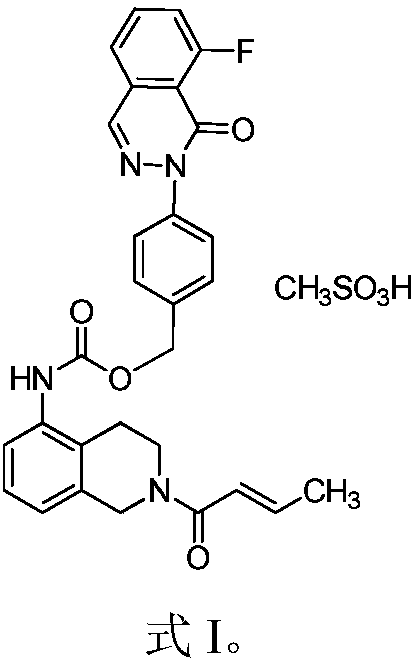
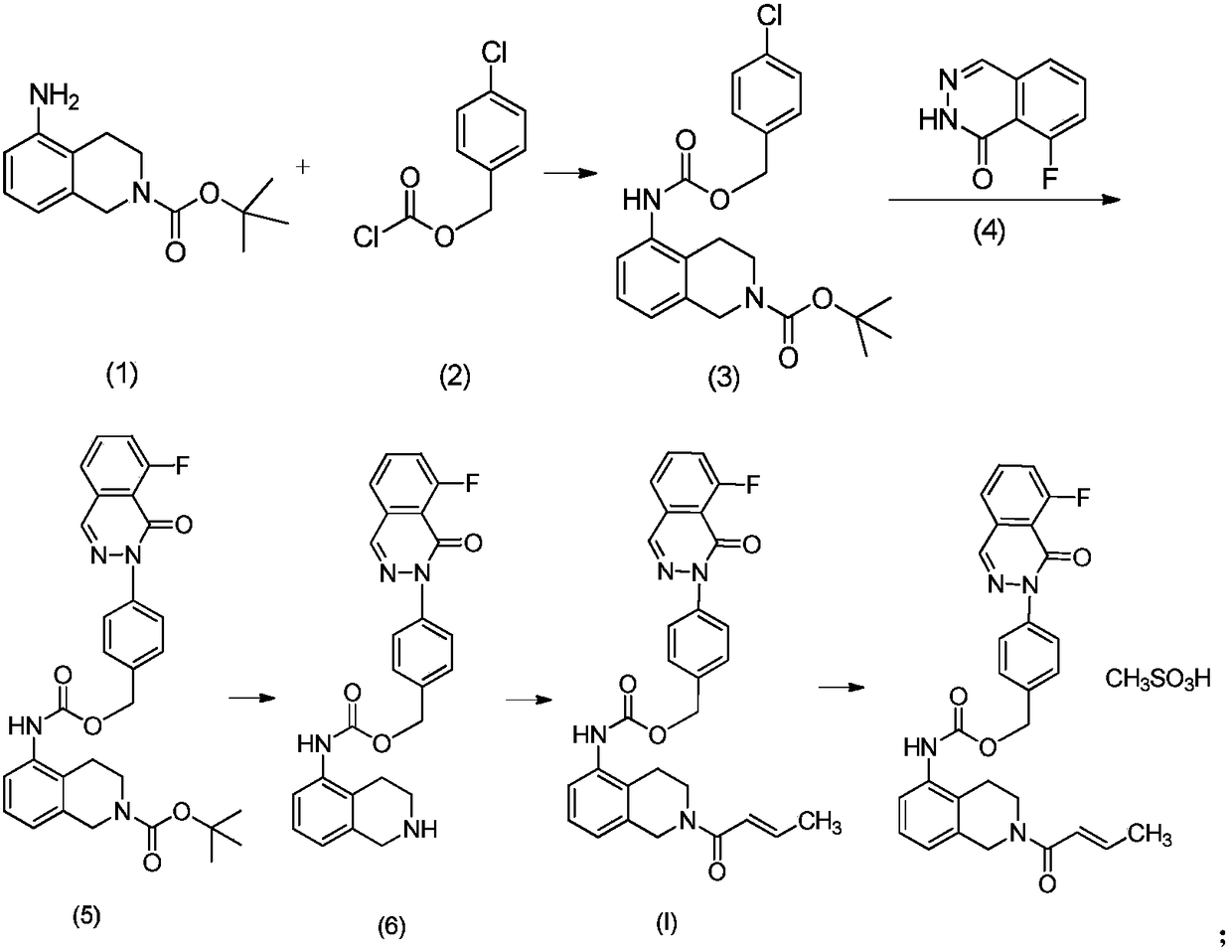
[0028] Example 1 [2(1H)-but-2-enoyl-3,4-dihydroisoquinolin-5-yl]-carbamic acid-4-[8-fluoro-(2H)-phthalazine-1- Preparation of keto]benzyl ester
[0029]
[0030] Step 1: Weigh 5-amino-3,4-dihydroisoquinoline-2(1H)-tert-butyl carboxylate (50mmol) and DIPEA (100mmol) in a reaction flask, add 300ml of dichloromethane, and stir at room temperature Slowly add p-chlorobenzyl chloroformate (51 mmol) dropwise, after the drop is complete, continue to stir at room temperature for 1 h, stop the reaction, concentrate the reaction mixture, add 70 ml of ethyl acetate, wash with dilute aqueous hydrochloric acid (0.2-0.3N) and saturated brine, Dry over anhydrous sodium sulfate, filter, and concentrate to give (3,4-dihydroisoquinoline-2(1H)-tert-butyl-5-yl carbamate)-p-chlorobenzyl carbamate, which is directly used in the next step, ESI –MS:[M+H] + m / z417.
[0031]Step 2: Weigh 3-fluoro-1-dimethoxymethylbenzene (500mmol) into a reaction flask, add tetrahydrofuran (800ml) to dissolve, add...
Embodiment 2
[0036] Example 2: [2(1H)-but-2-enoyl-3,4-dihydroisoquinolin-5-yl]-carbamic acid-4-[8-fluoro-(2H)-phthalazine-1 Preparation of -keto]benzyl ester methanesulfonate
[0037] Weigh the compound of Example 1 (0.5 mmol) into a reaction flask, add 5 mL of acetone and stir at room temperature for 0.5 h, add dropwise 1 mL of methanesulfonic acid acetone solution (containing 0.55 mmol of methanesulfonic acid), and continue stirring for 1 h at room temperature. The solvent was distilled off under pressure and dried under vacuum at room temperature to obtain the title compound.
[0038] 1 H NMR (600MHz, CDCl 3 )(δ,ppm):9.50(s,1H,disappeared after heavy water exchange),8.10(s,1H),7.88~7.85(m,1H),7.77~7.75(m,1H),7.54~7.52(m, 3H),7.34~7.32(m,2H),7.21~7.18(m,3H),6.57~6.55(m,1H),6.41~6.39(m,1H),5.17(s,2H),4.22(s, 2H), 3.61~3.59(m,2H), 3.13~3.11(m,2H), 2.84~2.81(s,3H), 2.05~2.03(m,3H).
Embodiment 3
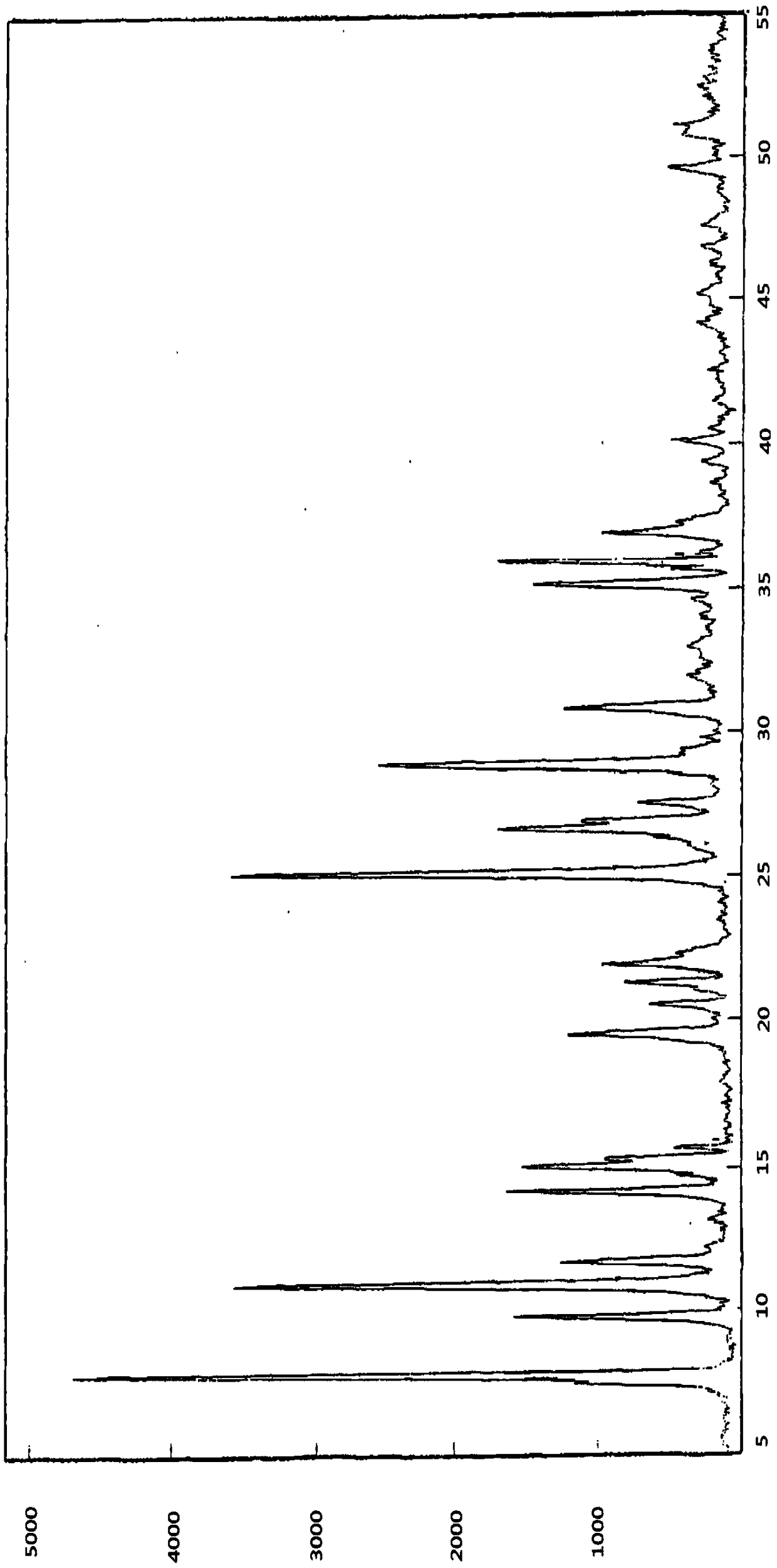
[0039] Example 3: [2(1H)-but-2-enoyl-3,4-dihydroisoquinolin-5-yl]-carbamic acid-4-[8-fluoro-(2H)-phthalazine-1 Preparation of N crystal form of -keto]benzyl ester methanesulfonate
[0040] Weigh 1.0g [2(1H)-but-2-enoyl-3,4-dihydroisoquinolin-5-yl]-carbamic acid-4-[8-fluoro-(2H)-phthalazine-1 -Keto]benzyl ester methanesulfonate in the reaction flask, add 5mL ethanol-dichloromethane-water mixed solution with a volume ratio of 15:5:3, reflux for 30min, cool naturally to room temperature, and crystallize at 0-5°C 12h, [2(1H)-but-2-enoyl-3,4-dihydroisoquinolin-5-yl]-carbamic acid-4-[8-fluoro-(2H)-phthalazine-1- Keto]benzyl ester methanesulfonate N crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com