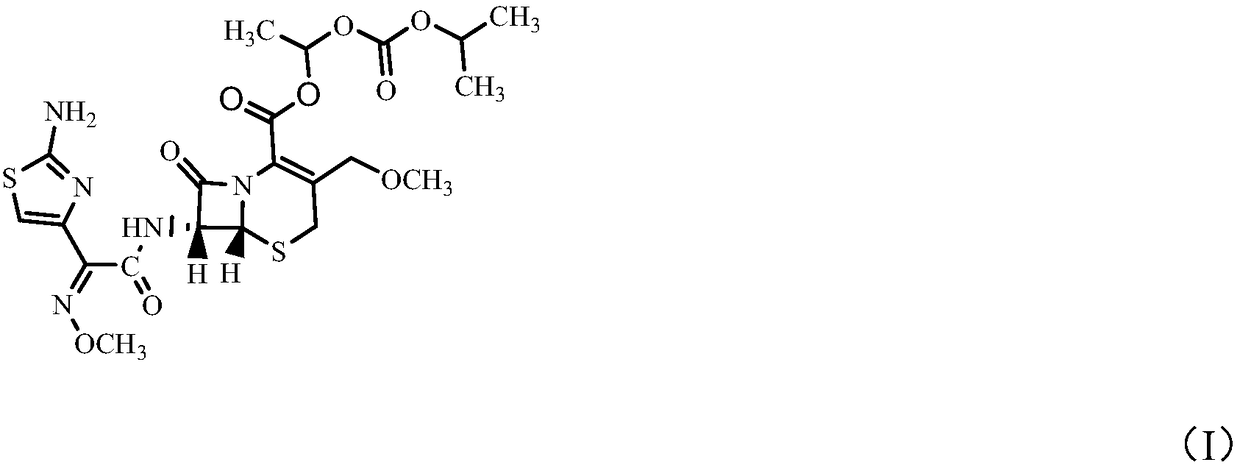
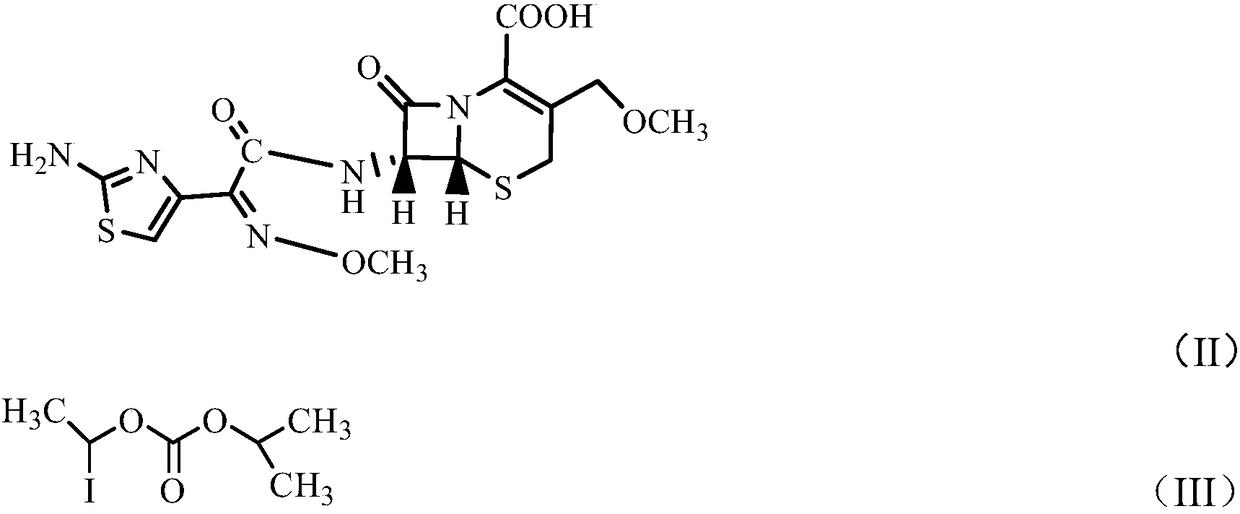
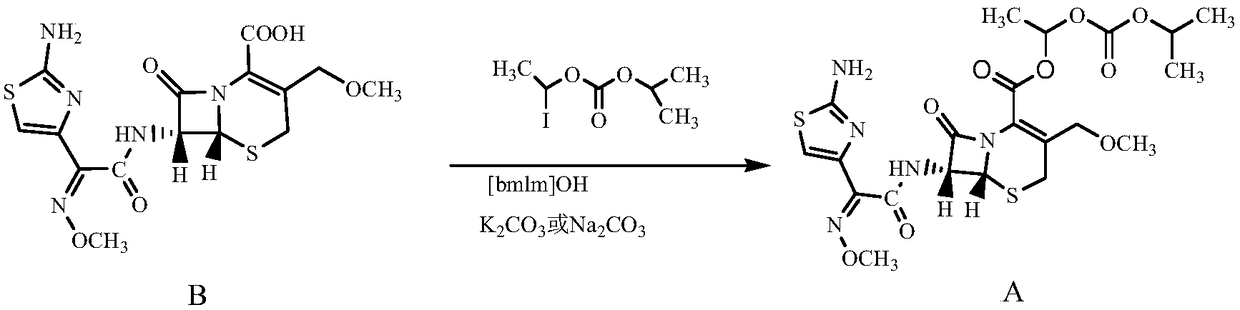
Method for preparing high-purity cefpodoxime proxetil
A technology of cefpodoxime axetil and cefpodoxime acid, which is applied in the field of preparing high-purity cefpodoxime axetil, can solve the problems of low yield and expensive quaternary ammonium salt, and achieve high yield, easy operation and good product stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 250ml reaction flask, put 10g (0.0234moL) of cefpodoxime acid B into 50ml of DMAc (N,N-dimethylacetamide) under nitrogen protection, stir and cool down to -20°C to dissolve, add [bmlm] OH0.05g, potassium carbonate 3.5g, stirred for 20 minutes. Add 6.5 g (0.0253 mol) of α-iodoethyl isopropyloxycarbonate dropwise, and control the temperature at -18°C to -19°C. After 30 minutes of reaction, the raw material cefpodoxamic acid B≤0.5% is controlled by HPLC, and the esterification reaction At the end, add 1g of medicinal activated carbon for decolorization. After decolorization, slowly press the esterification reaction product into 400ml of 1% methanol / purified aqueous solution that has been pre-cooled to 5°C. The product was washed with 50 mL of water and dried in vacuum to obtain 10.5 g of white powder solid (80.6% molar yield).
[0030] Product (R+S)HPLC=99.2%, R / (R+S)=0.51, Δ 3 = 0.35%.
Embodiment 2
[0032] In a 250ml reaction flask, put 10g (0.0234moL) of cefpodoxamic acid B into 50ml of DMAc under nitrogen protection, stir and cool down to -20°C to dissolve, add [bmlm]OH0.1g, sodium carbonate 3.1g and stir for 20 minutes . Add 6.5 g (0.0253 mol) of α-iodoethyloxyisopropyl carbonate dropwise, control the temperature at -18°C to -19°C, and react for 30 minutes.
[0033] HPLC control raw material cefpodoxamic acid B ≤ 0.5%, after the esterification reaction is completed, add 1g of medicinal activated carbon for decolorization, after the decolorization is completed, slowly press the esterification reactant into the pre-cooled to 5°C containing 1% methanol / purified aqueous solution In 400ml, after press filtration, continue to stir for 30 minutes, shake off the filter, wash the product with 50mL of purified water, and vacuum dry to obtain 10.2g of white powder solid (molar yield=78.3%).
[0034] Product (R+S)HPLC=99.1%, R / (R+S)=0.53, Δ3=0.36%.
Embodiment 3
[0036] Put 20g (0.0468moL) of cefpodoxamic acid B into 100ml of DMF in a 250ml four-necked flask under the protection of nitrogen, stir and cool down to -15℃~-18℃ to dissolve, add [bmlm]OH0.2g, potassium carbonate 6.3 g, stirred for 20 minutes. Add 13 g (0.0505 mol) of α-iodoethyl isopropyloxycarbonate dropwise, and keep the reaction for 30 minutes. After the HPLC control raw material cefpodoxamic acid B≤0.5%, the esterification reaction is completed, add 2 g of medicinal active carbon for decolorization, decolorization Slowly press the esterification reactant into 800 ml of 2% ethanol / purified aqueous solution pre-cooled to 5° C., press filter and precipitate solid, and continue to stir for 30 minutes. It was filtered by rejection, washed with 50 mL of purified water, and dried in vacuum to obtain 18.9 g of white powder solid (weight yield=94.5%).
[0037] Product (R+S)HPLC=99.2%, R / (R+S)=0.51, Δ 3 = 0.37%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com