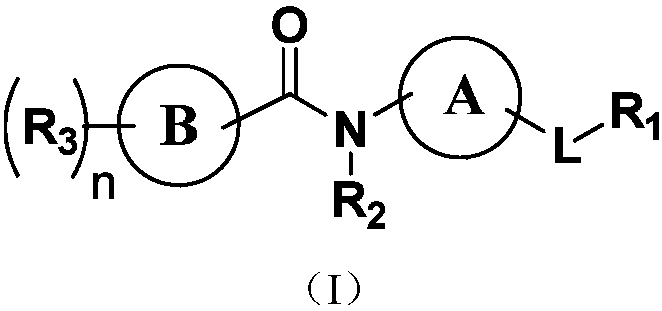
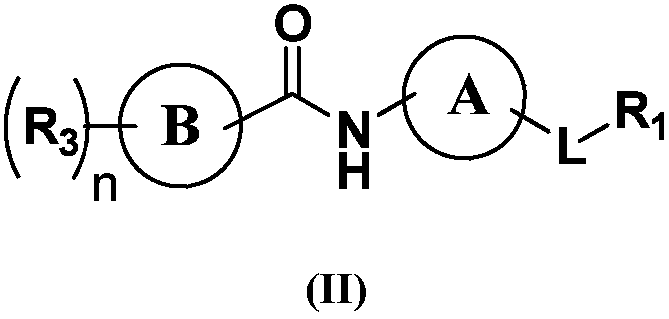
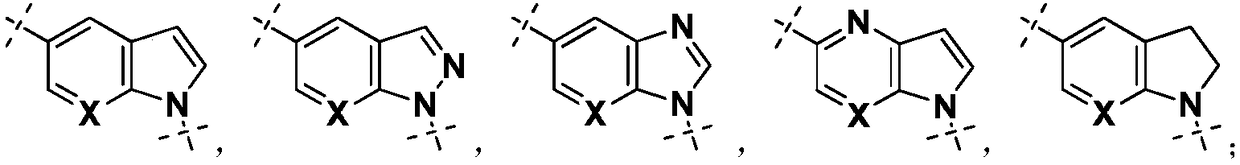
Indole derivatives or salts thereof as well as preparation method and application of indole derivatives or salts thereof
A kind of technology of indoles and derivatives, applied in the field of chemical medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1 Preparation of N-(1-benzoyl-1H-indol-6-yl)-2,5-dichlorobenzamide
[0102] (N-(1-benzoyl-1H-indol-5-yl)-2,5-dichlorobenzamide)
[0103]
[0104] Step 1: Synthesis of (5-nitro-1H-indol-1-yl)(phenyl)methanone
[0105] Benzoic acid (293mg, 2.39mmol, 1.2equiv.), HATU (912mg, 2.40mmol, 1.3equiv.), DIPEA (310mg, 2.40mmol, 1.3equiv.) were dissolved in an appropriate amount of DCM, stirred for 10 minutes and then added 5-nitro -1H-indole (324 mg, 2.00 mmol, 1.0 equiv.), stirred at room temperature for 8 hours. TLC monitored the reaction in real time. After the reaction is complete, dilute with water, extract three times with an appropriate amount of ethyl acetate, combine the organic phases, and successively dilute with water, saturated NaHCO 3 solution, saturated NaCl solution, and the organic layer was washed with anhydrous NaSO 4 Dry and distill under reduced pressure to obtain a crude product, which is purified on a silica gel column to obtain (5-nitro-1H-in...
Embodiment 2
[0111] Example 2 Preparation of N-(1-benzoyl-1H-indazol-5-yl)-2,5-dichlorobenzamide
[0112] (N-(1-benzoyl-1H-indazol-5-yl)-2,5-dichlorobenzamide)
[0113]
[0114] Step 1: Synthesis of 1H-indazol-5-amine
[0115] 5-Nitro-1H-indazole (430 mg, 1.0 equiv.) was dissolved in ethyl acetate, a catalytic amount of Pd / C (40 mg, 10% w / w) was added under nitrogen protection, replaced by hydrogen, and stirred at room temperature for 1.5 hours. TLC monitored the reaction in real time. After the reaction was completed, the reaction solution was filtered through diatomaceous earth, washed with an appropriate amount of methanol, and the organic phase was distilled under reduced pressure to obtain the crude product 1H-indazol-5-amine (330 mg, yield: 94.3%).
[0116] Step 2: Synthesis of 2,5-dichloro-N-(1H-indazol-5-yl)benzamide
[0117] 1H-Indazol-5-amine (130mg, 1.0equiv.), K 2 CO 3 (165mg, 1.2equiv.) dissolved in anhydrous CH 3 CN, newly prepared 2,5-dichlorobenzoyl chloride (250 m...
Embodiment 3
[0121] Example 3 Preparation of N-(1-benzoyl-1H-benzo[d]imidazol-5-yl)-2,5-dichlorobenzamide
[0122] (N-(1-benzoyl-1H-benzo[d]imidazol-5-yl)-2,5-dichlorobenzamide)
[0123]
[0124] Step 1: Synthesis of (5-nitro-1H-benzo[d]imidazol-1-yl)(phenyl)methanone
[0125] 5-nitro-1H-benzo[d]imidazole (820mg, 5.03mmol, 1.0equiv.), Et 3 N (560mg, 5.53mmol, 1.1equiv.) was dissolved in an appropriate amount of DMF, and newly prepared benzoyl chloride (770mg, 5.47mmol, 1.1equiv.) was dissolved in anhydrous DCM, slowly added dropwise at 0°C, and stirred for 4 hours under nitrogen protection. TLC monitored the reaction in real time. After the reaction is complete, dilute with water, extract three times with an appropriate amount of ethyl acetate, combine the organic phases, and successively dilute with water, saturated NaHCO 3 solution, saturated NaCl solution, and the organic layer was washed with anhydrous NaSO 4 Dry and distill under reduced pressure to obtain a crude product, whic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com