A kind of synthetic method of 2-aminobenzophenone compounds
A technology of aminobenzophenone and aniline compounds, which is applied in the field of synthesis of 2-aminobenzophenone compounds, and achieves the effects of low energy consumption, mild reaction conditions, economy and good regioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016]
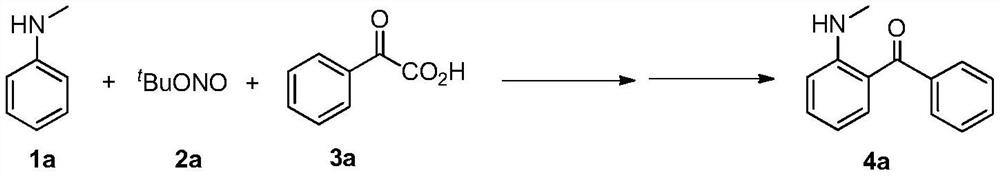
[0017] Add N-methylaniline (1a, 0.5mmol, 67mg), 1,2-dichloroethane (2mL), tert-butyl nitrite (2a, 1.5mmol, 180μL) and palladium acetate to a 25mL pressure tube in sequence (0.025mmol, 5.6mg) and benzoylformic acid (3a, 1mmol, 150mg), the pressure tube was sealed and placed in a reactor, stirred and reacted at 40°C for 20 hours. Then iron powder (2mmol, 112mg) and ammonium chloride (1.5mmol, 80mg) were directly added to the above reaction system, and reacted at 80°C for 10 hours. After the reaction, saturated sodium chloride solution (5mL) and ethyl acetate were added ester (8mL), then filtered, the filtrate was extracted with ethyl acetate (5mL×2), the organic phases were combined, dried with anhydrous sodium sulfate, the solvent was recovered by distillation under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum ether / acetic acid Ethyl ester=30 / 1, v / v), the product 4a was obtained as a yellow solid (71 mg, 67%). The ch...
Embodiment 2
[0019] Add N-methylaniline (1a, 0.5mmol, 67mg), 1,2-dichloroethane (2mL), tert-butyl nitrite (2a, 1.5mmol, 180μL) and palladium acetate to a 25mL pressure tube in sequence (0.025mmol, 5.6mg) and benzoylformic acid (3a, 1mmol, 150mg), the pressure tube was sealed and placed in a reactor, stirred and reacted at 30°C for 20 hours. Then iron powder (2mmol, 112mg) and ammonium chloride (1.5mmol, 80mg) were directly added to the above reaction system, and reacted at 80°C for 10 hours. After the reaction, saturated sodium chloride solution (5mL) and ethyl acetate were added ester (8mL), then filtered, the filtrate was extracted with ethyl acetate (5mL×2), the organic phases were combined, dried with anhydrous sodium sulfate, the solvent was recovered by distillation under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum ether / acetic acid Ethyl ester=30 / 1, v / v), the product 4a was obtained as a yellow solid (61 mg, 58%).
Embodiment 3
[0021] Add N-methylaniline (1a, 0.5mmol, 67mg), 1,2-dichloroethane (2mL), tert-butyl nitrite (2a, 1.5mmol, 180μL) and palladium acetate to a 25mL pressure tube in sequence (0.025mmol, 5.6mg) and benzoylformic acid (3a, 1mmol, 150mg), the pressure tube was sealed and placed in a reactor, stirred and reacted at 50°C for 20 hours. Then iron powder (2mmol, 112mg) and ammonium chloride (1.5mmol, 80mg) were directly added to the above reaction system, and reacted at 80°C for 10 hours. After the reaction, saturated sodium chloride solution (5mL) and ethyl acetate were added ester (8mL), then filtered, the filtrate was extracted with ethyl acetate (5mL×2), the organic phases were combined, dried with anhydrous sodium sulfate, the solvent was recovered by distillation under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum ether / acetic acid Ethyl ester=30 / 1, v / v), the product 4a was obtained as a yellow solid (69 mg, 65%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com