A method for synthesizing methylheptenone by isopentenol
A technology of methyl heptenone and prenol, which is applied in chemical instruments and methods, preparation of organic compounds, carbon-based compounds, etc., can solve problems such as complicated side reactions, difficult product purification, and excessive waste, and achieve Effect of reducing reaction time, increasing condensation reaction yield, and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
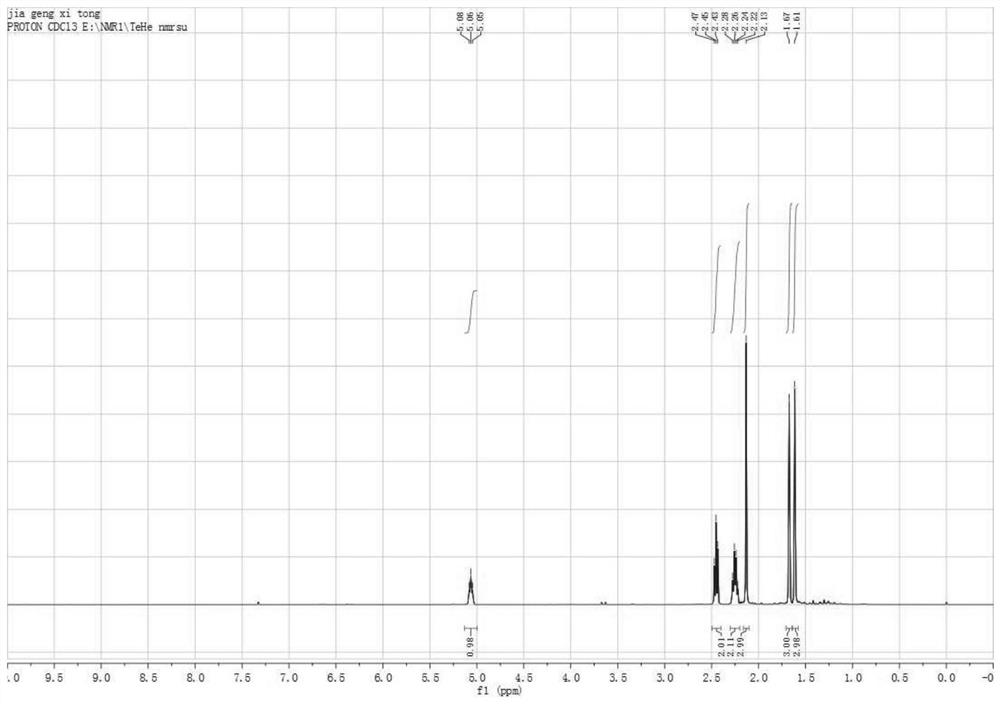
Image
Examples
Embodiment 1
[0048] This example catalyzes the chlorination of prenol by zinc chloride to synthesize methylheptenone in N,N-dimethylformamide.
[0049]Under nitrogen protection, 60.0 mL of concentrated hydrochloric acid (38%, 0.74 mol), 0.27 g of zinc chloride (0.002 mol) and 17.2 g of prenyl alcohol (0.2 mol) were successively added into the three-necked flask. Put in a magnetic stirrer and assemble a reflux condenser, put the three-neck flask in an oil bath at 50°C, stir the reaction rapidly, and the reaction solution gradually becomes turbid from a homogeneous phase. After 3 hours, GC detected the organic phase, and the conversion rate of prenol was >98%. The reaction was stopped, the temperature was lowered, and the phases were separated. After washing the organic phase with saturated aqueous sodium bicarbonate solution and deionized water, the product 19.5 g, yield 92.2%. Product composition: 1-chloro-3-methyl-2-butene (91.1 wt%), 3-chloro-3-methyl-1-butene (7.6 wt%), dichloride (1.3...
Embodiment 2
[0053] This example catalyzes the chlorination of isopentenol by zinc chloride to synthesize methylheptene in N,N-dimethylformamide.
[0054] Under the protection of nitrogen, concentrated hydrochloric acid (81.4 mL, 38%, 1.0 mol), zinc chloride (27.3 mg, 0.0002 mol) and isopentenol (17.2 g, 0.2 mol) were successively added into the three-necked flask. Put a magnetic stirrer in the three-necked flask, and after it is equipped with a reflux condenser, put the three-necked flask in an oil bath at 40°C, and react under rapid stirring. During the reaction, the reaction solution gradually becomes turbid from a homogeneous phase. After 1 hour, GC detects the organic phase, and the conversion rate of prenol is >98%. Stop the reaction, lower the temperature, separate the liquid using a separatory funnel, wash the organic phase with saturated aqueous sodium bicarbonate solution and deionized water successively, and distill under reduced pressure to obtain Product 19.2g, yield 90.0%. P...
Embodiment 3
[0057] This example catalyzes the chlorination of prenol by zinc chloride to synthesize methylheptenone in N,N-dimethylformamide.
[0058] Under nitrogen protection, concentrated hydrochloric acid (62.0 mL, 10%, 0.2 mol), zinc chloride (2.7 g, 0.02 mol) and isopentenol (17.2 g, 0.2 mol) were sequentially added into the three-necked flask. Put a magnetic stirrer in the three-necked flask, and after it is equipped with a reflux condenser, put the three-necked flask in an oil bath at 70°C, and react under rapid stirring. During the reaction, the reaction solution gradually becomes turbid from a homogeneous phase. After 3 hours, GC detected the organic phase, and the conversion rate of prenol was >98%. Stop the reaction, lower the temperature, separate the liquid using a separatory funnel, wash the organic phase with saturated aqueous sodium bicarbonate solution and deionized water successively, and distill under reduced pressure to obtain Product 17.6g, yield 83.3%. Product comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com