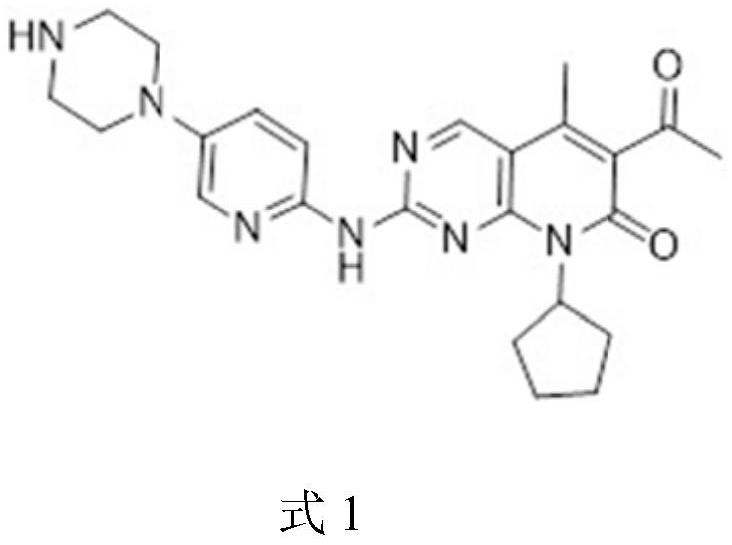
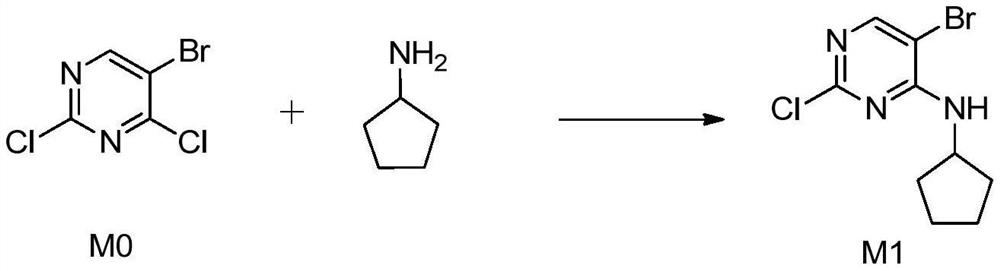
A kind of preparation technology of Palbocoxib intermediate 5-bromo-2-chloro-4-cyclopentylaminopyrimidine
A preparation process and intermediate technology, applied in the field of intermediate M1 and palbocoxib, to achieve significant effects, improve the production process, and reduce the generation of substitution impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 2,4-Dichloro-5-bromopyrimidine M0 (200g, 888mmol) and 1.4L dichloromethane were added to the reaction flask, sodium bicarbonate (372g, 4440mmol) was added, and 0.6L dichloromethane was slowly added dropwise at room temperature. Cyclopentylamine (90.7g, 1066mmol) diluted with chloromethane was reacted at room temperature for 13h, the sodium bicarbonate and the resulting inorganic salt were removed by suction filtration, the mother liquor was rotary evaporated to remove dichloromethane, and 1.0L of n-heptane was added for recrystallization to obtain a white solid 236.7g, M1 yield 97.5%, purity >99.8%, impurity 5-bromo-4-chloro-2-cyclopentylaminopyrimidine <0.02%. Melting point: 94-96°C.
[0022] 1H NMR(DMSO-d6)δ(ppm): 8.23(1H,s), 7.35(1H,d), 4.40–4.22(1H,m), 1.98–1.84(2H,m), 1.75–1.66(2H, m), 1.64–1.50 (4H, m).
Embodiment 2
[0024] 2,4-Dichloro-5-bromopyrimidine M0 (100g, 444mmol) and 1.0L methyl tert-butyl ether were added to the reaction flask, potassium bicarbonate (444g, 4440mmol) was added, and at room temperature, slowly added dropwise with 0.3L cyclopentylamine (45.4g, 533mmol) diluted with methyl tert-butyl ether was reacted at room temperature for 12h, the potassium bicarbonate and the resulting inorganic salt were removed by suction filtration, the mother liquor was rotary evaporated to remove the methyl tert-butyl ether, and 0.5L was added. The n-heptane was recrystallized to obtain 117.9 g of white solid, the yield of M1 was 97.1%, the purity was >99.8%, and the impurity 5-bromo-4-chloro-2-cyclopentylaminopyrimidine was less than 0.02%. Melting point: 94-96°C.
[0025] 1H NMR(DMSO)δ(ppm): 8.23(1H,s), 7.35(1H,d), 4.40-4.22(1H,m), 1.98-1.84(2H,m), 1.75-1.66(2H,m) , 1.64–1.50 (4H, m).
Embodiment 3
[0027] 2,4-Dichloro-5-bromopyrimidine M0 (100g, 444mmol) and 1.0L dichloromethane were added to the reaction flask, potassium bicarbonate (444g, 4440mmol) was added, and 0.3L dichloromethane was slowly added dropwise at room temperature. Cyclopentylamine (45.4g, 533mmol) diluted with chloromethane was reacted at room temperature for 15h, the potassium bicarbonate and the resulting inorganic salt were removed by suction filtration, the mother liquor was rotary evaporated to remove dichloromethane, and 0.5L of n-heptane was added for recrystallization to obtain a white solid 117.5g, M1 yield 96.8%, purity >99.8%, impurity 5-bromo-4-chloro-2-cyclopentylaminopyrimidine <0.02%. Melting point: 94-96°C.
[0028] 1H NMR(DMSO)δ(ppm): 8.23(1H,s), 7.35(1H,d), 4.40-4.22(1H,m), 1.98-1.84(2H,m), 1.75-1.66(2H,m) , 1.64–1.50 (4H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com