Preparation method of indium oxide with controllable crystal form
An indium oxide, crystal form technology, applied in chemical instruments and methods, inorganic chemistry, gallium/indium/thallium compounds, etc., can solve the problems of difficult industrial application, difficult adjustment, discontinuous reaction process, etc. The effect of mass transfer efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
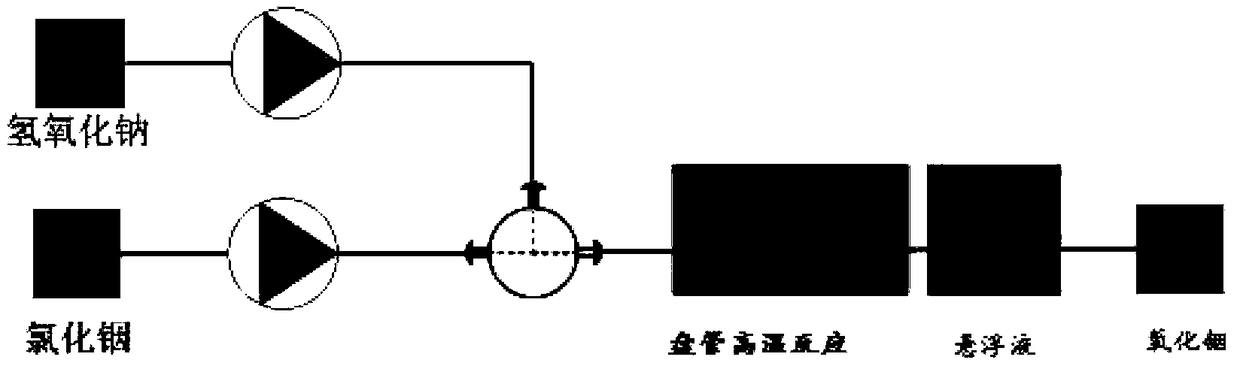
[0020] The invention provides a method for preparing indium oxide with controllable crystal form, comprising the following steps:
[0021] In the microreactor, the indium chloride solution and the sodium hydroxide solution are mixed for a precipitation reaction to obtain a precipitated product, and the precipitated product is subjected to a high-temperature reaction in a coil to obtain an indium oxide precursor suspension;
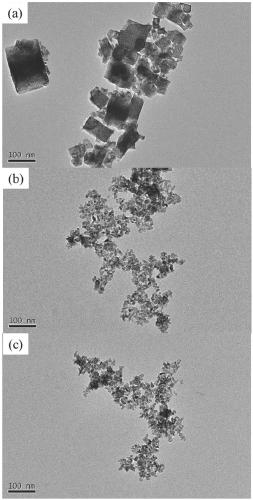
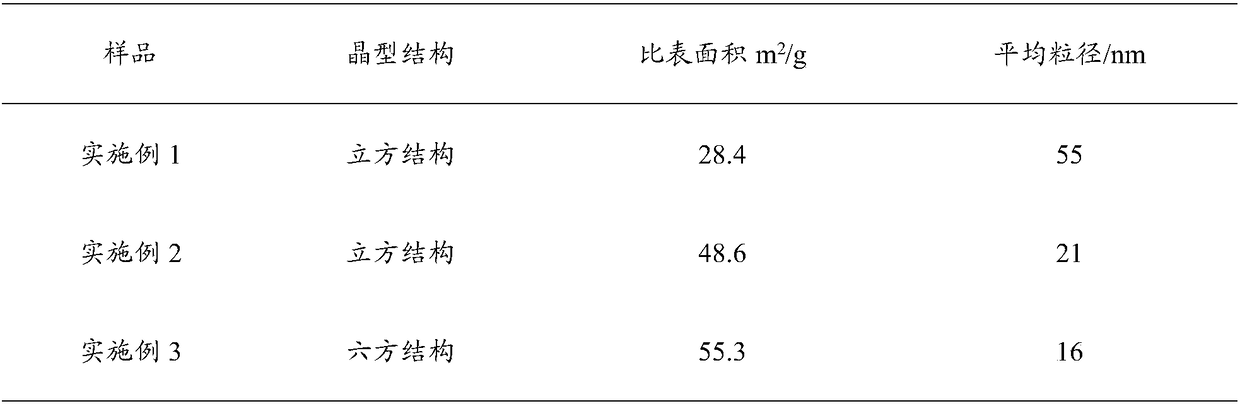
[0022] The indium oxide precursor suspension is sequentially separated, washed, dried, and high-temperature roasted to obtain indium oxide; when the temperature of the high-temperature reaction is [100,140]°C, the obtained indium oxide has a cubic structure, and when the high-temperature reaction When the reaction temperature is [160,180]°C, the obtained indium oxide has a hexagonal structure.
[0023] In the present invention, in a microreactor, an indium chloride solution and a sodium hydroxide solution are mixed for a precipitation reaction to obtain a ...
Embodiment 1
[0035] 1) Prepare an indium chloride solution with a concentration of 1g / L, and make it flow into the microreactor at a flow rate of 5mL / min.
[0036] 2) Prepare a sodium hydroxide aqueous solution with a concentration of 2mol / L, and make it flow into the microreactor at a flow rate of 5mL / min, realize rapid mixing of the two phases by shearing the dispersed phase, and pass it into the subsequent coil.
[0037] 3) Pass the mixed solution through a 16-meter-long coil pipe to continue the reaction at 140° C., and the reacted mixed solution is collected in a stirred tank after being cooled.
[0038] 4) Centrifuge the mixed liquid in the stirred tank, wash the obtained solid twice with deionized water and ethanol, and then dry to obtain the precursor powder.
[0039] 5) Put the precursor powder in a muffle furnace and bake it at 400°C to obtain a yellow indium oxide powder, denoted as a.
Embodiment 2
[0041] 1) Prepare an indium chloride solution with a concentration of 1g / L, and make it flow into the microreactor at a flow rate of 10mL / min.
[0042] 2) Prepare a sodium hydroxide aqueous solution with a concentration of 1mol / L, and make it flow into the microreactor at a flow rate of 10mL / min, realize rapid mixing of the two phases by shearing the dispersed phase, and pass it into the subsequent coil.
[0043] 3) Pass the mixed solution through an 8-meter-long coil pipe to continue the reaction at 100° C., and the reacted mixed solution is collected in a stirred tank after being cooled.
[0044] 4) Centrifuge the mixed liquid in the stirred tank, wash the obtained solid twice with deionized water and ethanol, and then dry to obtain the precursor powder.
[0045] 5) Put the precursor powder in a muffle furnace and bake it at 400°C to obtain a yellow indium oxide powder, denoted as b.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com