Markers for evaluating risk of familial breast cancer and application thereof
A breast cancer, marker technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1 Sample collection and clinical data collection of hereditary breast cancer in different families
[0085] 1. Inclusion criteria of families with breast cancer patients
[0086] 1) In a family, except the proband, there is one or more cases of breast cancer in the first-degree relatives, and at least one case meets one of the following conditions: <40 years old at onset; bilateral breast cancer at the same time or successively Breast cancer; non-breast malignancies concurrently or sequentially;
[0087] 2) Have not received any relevant treatment before surgery, such as radiotherapy, chemotherapy, endocrine therapy and targeted therapy;
[0088] 3) Those who meet the international clinical stage I-IV without contraindications, who have undergone relevant surgical treatment (including mastectomy for breast cancer surgery, radical mastectomy, modified radical mastectomy, and breast-conserving surgery, with or without axillary surgery) lymphadenectomy).
[0089...
Embodiment 2
[0098] Example 2 Using iTRAQ technology to carry out blood proteomics research identification analysis
[0099] 1. Test samples
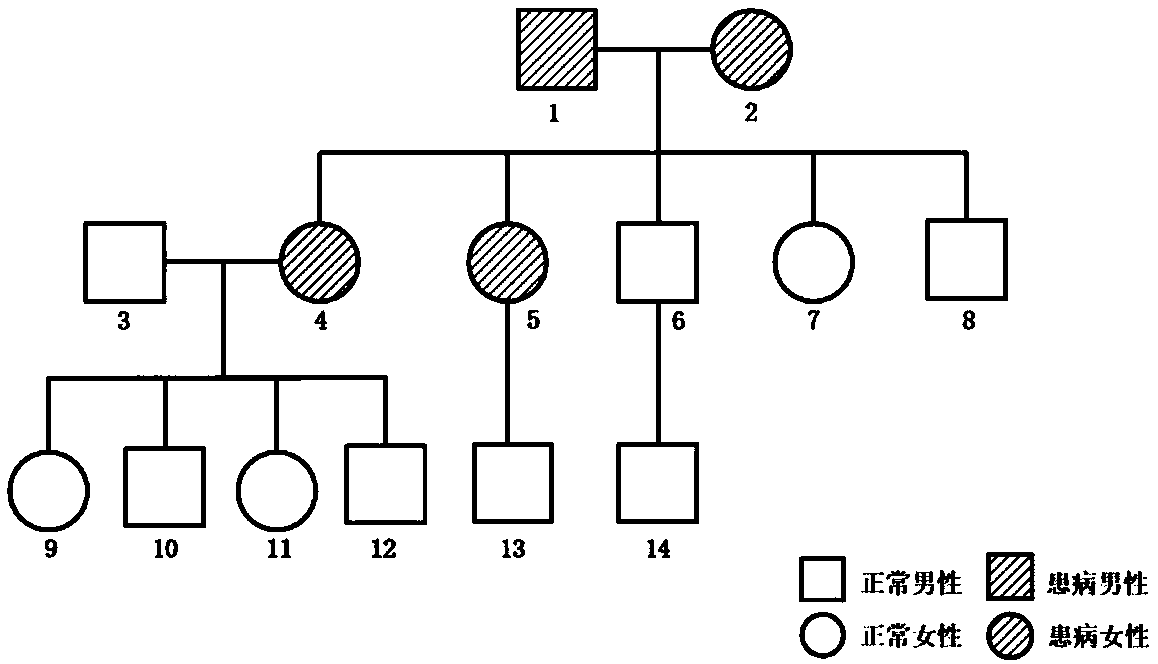
[0100] Family 1 ( figure 1Family 1 map) includes 14 members, of which No. 1 died, No. 2 was a patient, family members No. 4 and No. 5 were sick, and the other 10 members were not sick; 4 people (1, 2, 4, 5 No.) suffered from malignant tumors, including breast cancer, pancreatic cancer, cervical cancer, thyroid cancer and liver cancer; 1 person (No. 4) suffered from three malignant tumors (breast cancer, thyroid cancer and liver cancer) at the same time. Among them, there were 3 cases (No. 2, No. 4, and No. 5) in the case group, and 10 cases (other members) in the control group. The blood of all members of the family was collected, left at 4°C for 1 hour, centrifuged at 3000g for 10 minutes, and the supernatant was collected and stored on ice. Aliquot and store at -80°C for later use.
[0101] 2. iTRAQ quantitative experiment
[0102] 2.1 Sample ...
Embodiment 3
[0149] Example 3 Whole Exome Sequencing
[0150] Beijing Novogene Technology Co., Ltd. uses Agilent's liquid-phase chip capture system to efficiently enrich human DNA from the entire exon region, and then perform high-throughput and high-depth sequencing on the Illumina Hiseq platform. All 3 patients and 10 control samples were from family 1.
[0151] 1) Genomic DNA was extracted from peripheral blood;
[0152] 2) Randomly break into fragments with a length of 180-280bp, and after end repair and A-tailing, link adapters at both ends of the fragments to prepare DNA libraries;
[0153] 3) Using Agilent's liquid phase chip capture kit (Agilent SureSelect Human All ExonV5 kit) to efficiently enrich human DNA from the entire exon region;
[0154] 4) Liquid-phase hybridization with up to 543,872 biotin-labeled probes after library pooling with a specific index;
[0155] 5) Using magnetic beads with streptomycin to capture 334,378 exons of 20,965 genes;
[0156] 6) Perform librar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com