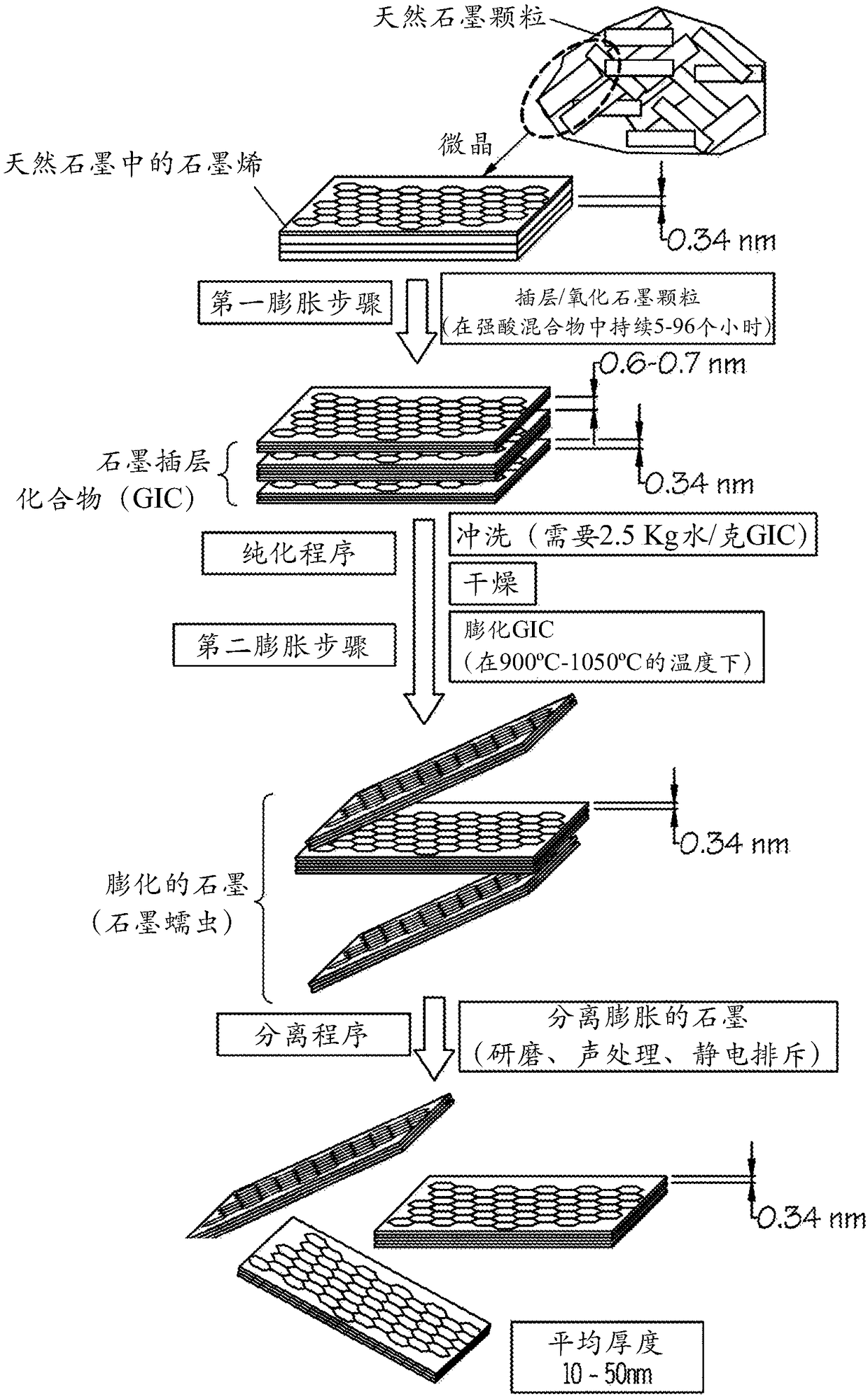
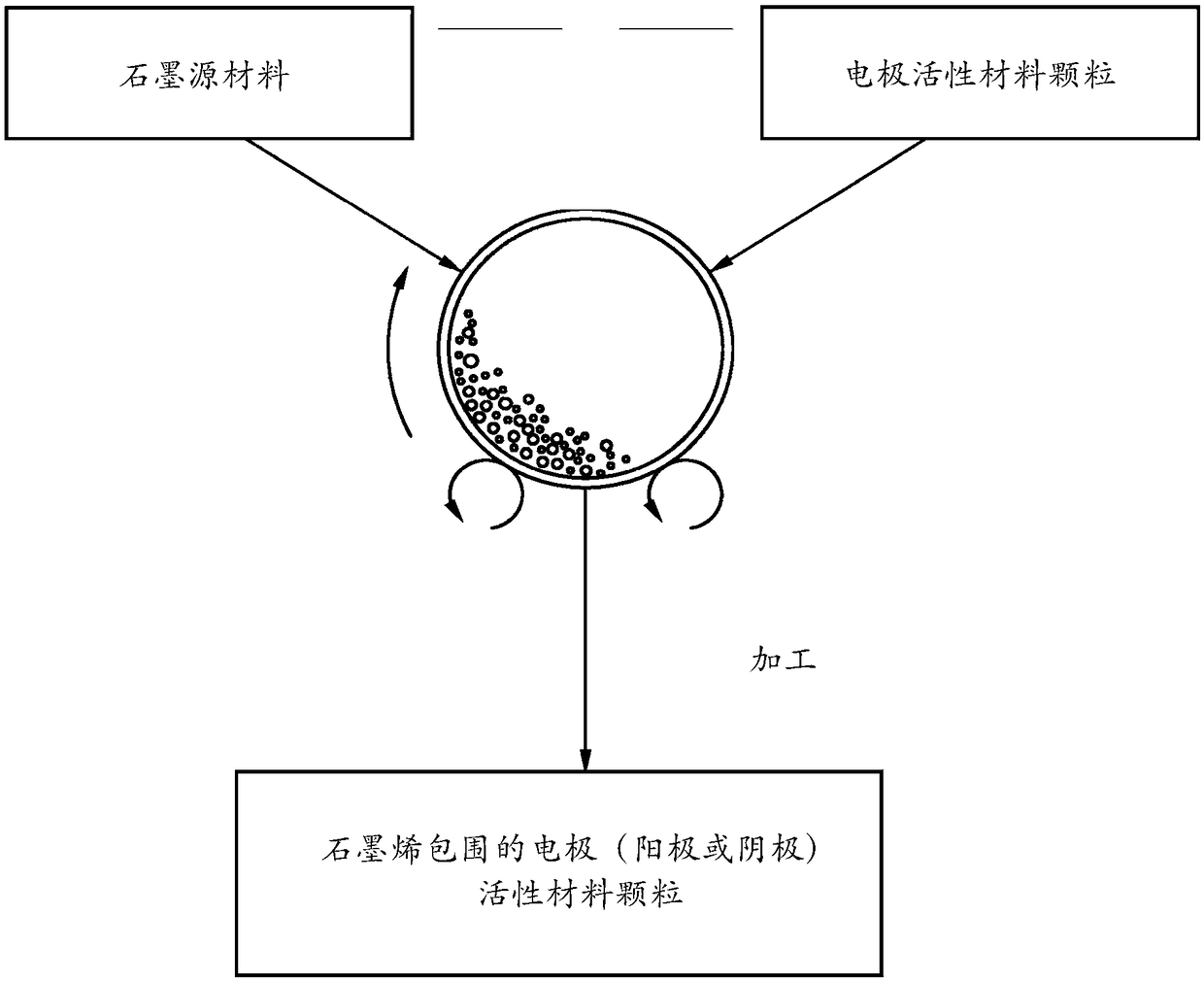
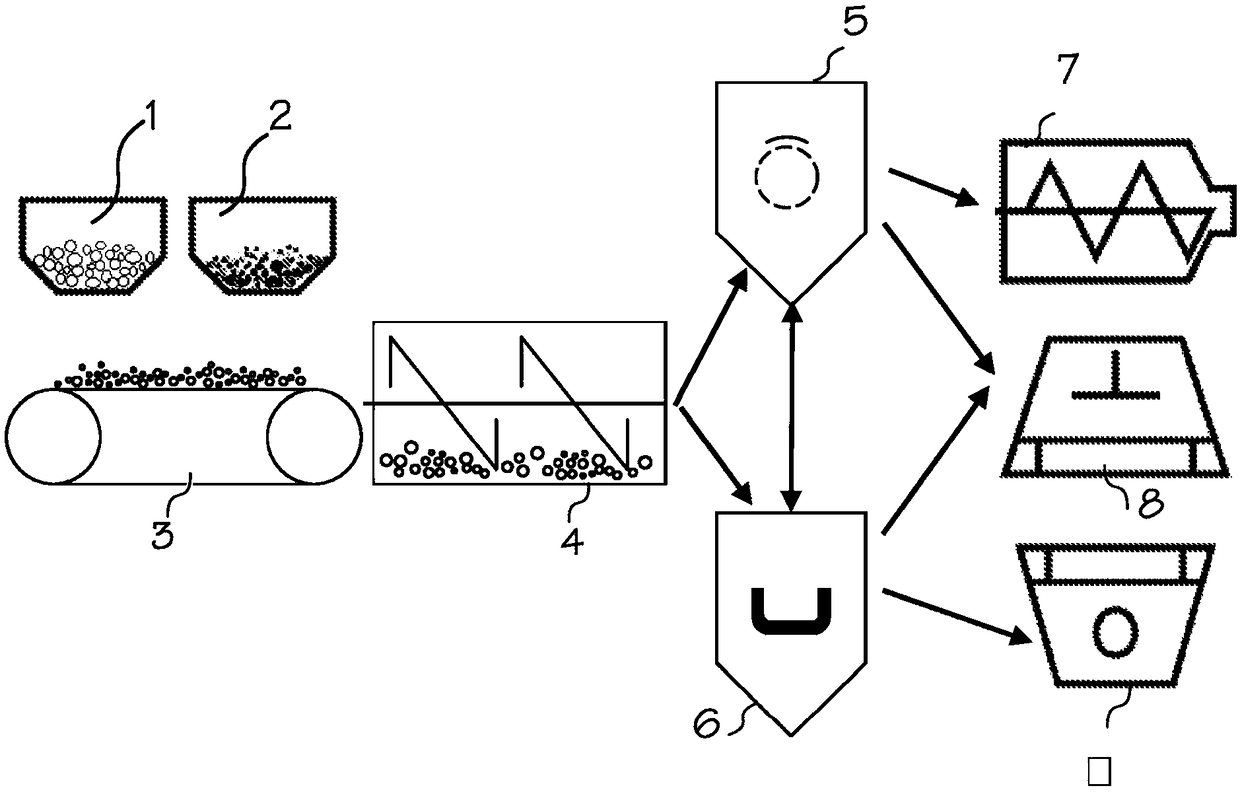
Chemical-free production of graphene-encapsulated electrode active material particles for battery applications
A technology of electrode active materials and graphite materials, applied in battery electrodes, electrochemical generators, non-aqueous electrolyte battery electrodes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0152] Example 1: Electrode active material particles surrounded by graphene
[0153] Several types of electrode active materials (both anode active materials and cathode active materials) in the form of fine powders have been investigated. These include Co 3 o 4 , Si, LiCoO 2 , LiMn 2 o 4 , Lithium Iron Phosphate, etc., as examples demonstrating best-practice patterns. These active materials are self-prepared or commercially available.
[0154] In a typical experiment, 1 kg of electrode active material powder and 100 g of natural flake graphite, 50 mesh (average particle size 0.18 mm; Asbury Carbons, Asbury NJ) in a high energy ball mill container. The ball mill was run at 300 rpm for 0.5 to 4 hours. The container lid was then removed and the active material particles were found to be completely coated (surrounded or encapsulated) by a dark layer, confirmed by Raman spectroscopy to be graphene. Blocks of processed material were placed on a 50 mesh screen and, in some...
example 2
[0155] Example 2: Functionalized graphene-encapsulated Sn particles
[0156] The procedure of Example 1 was repeated, including 50 g of urea as nitrogen source. The resulting coated powders are functionalized graphene-encapsulated Sn particles used as anode active materials in Li-ion batteries. It can be noted that chemical functionalization is used to improve the wettability of the electrode active material by the electrolyte or the compatibility between the electrode active material and the electrolyte in the battery.
example 3
[0157] Example 3: SnO Surrounded by Graphene 2 particles
[0158] In the experiment, 2 g of 99.9% pure tin oxide powder (90 nm diameter) and 0.25 g of highly oriented pyrolytic graphite (HOPG) were placed in a resonant acoustic mill and processed for 5 minutes. For comparison, the same experiment was performed, but the grinding vessel further contained zirconia grinding beads. We unexpectedly found that the former approach (tin oxide particles themselves acting as grinding media without externally added zirconia grinding beads) resulted in predominantly single-particle microparticles (each containing one particle encapsulated by a graphene sheet). In contrast, in the presence of externally added grinding beads, graphene-surrounded microparticles tended to contain multiple tin oxide particles (typically 3-50) surrounded by graphene sheets. These same results were also observed for most metal oxide based electrode active materials (both anode and cathode). We further observe ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com