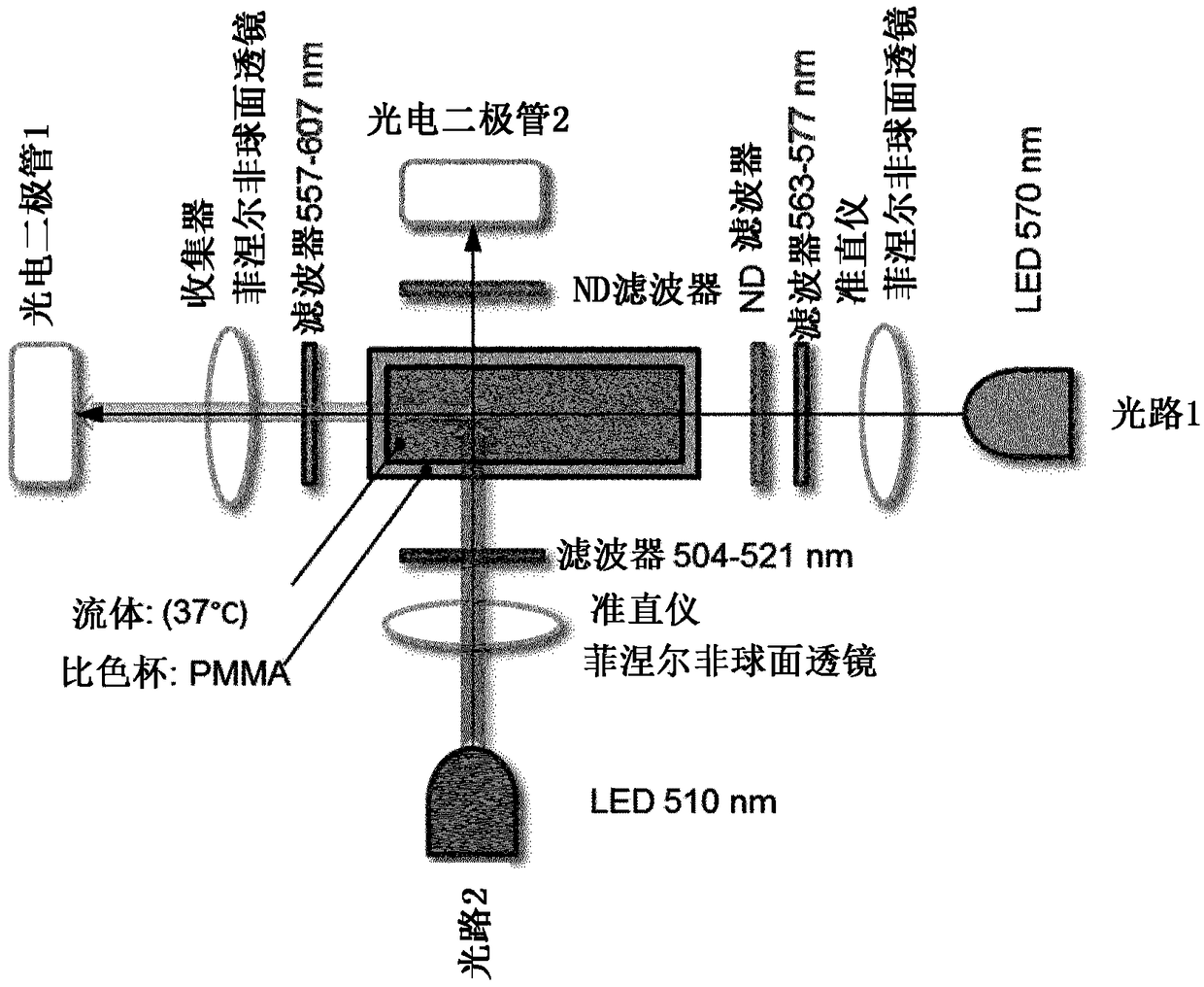
Method for determining the quantity of an hba1c in a blood sample
A technology of blood samples and liquids, applied in the field of volume suits, can solve the problems of cost reduction and increase of ease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0096] Example 1. HbA1c assay and calibration process
[0097] sample
[0098] Six calibrator samples (all representing different levels of HbAlc concentration in human blood) were purchased from the Lyphochek Hemoglobin Alc Linear Set (BIO-RAD). Six samples from this BIO-RAD kit were lysed according to the manufacturer's instructions.
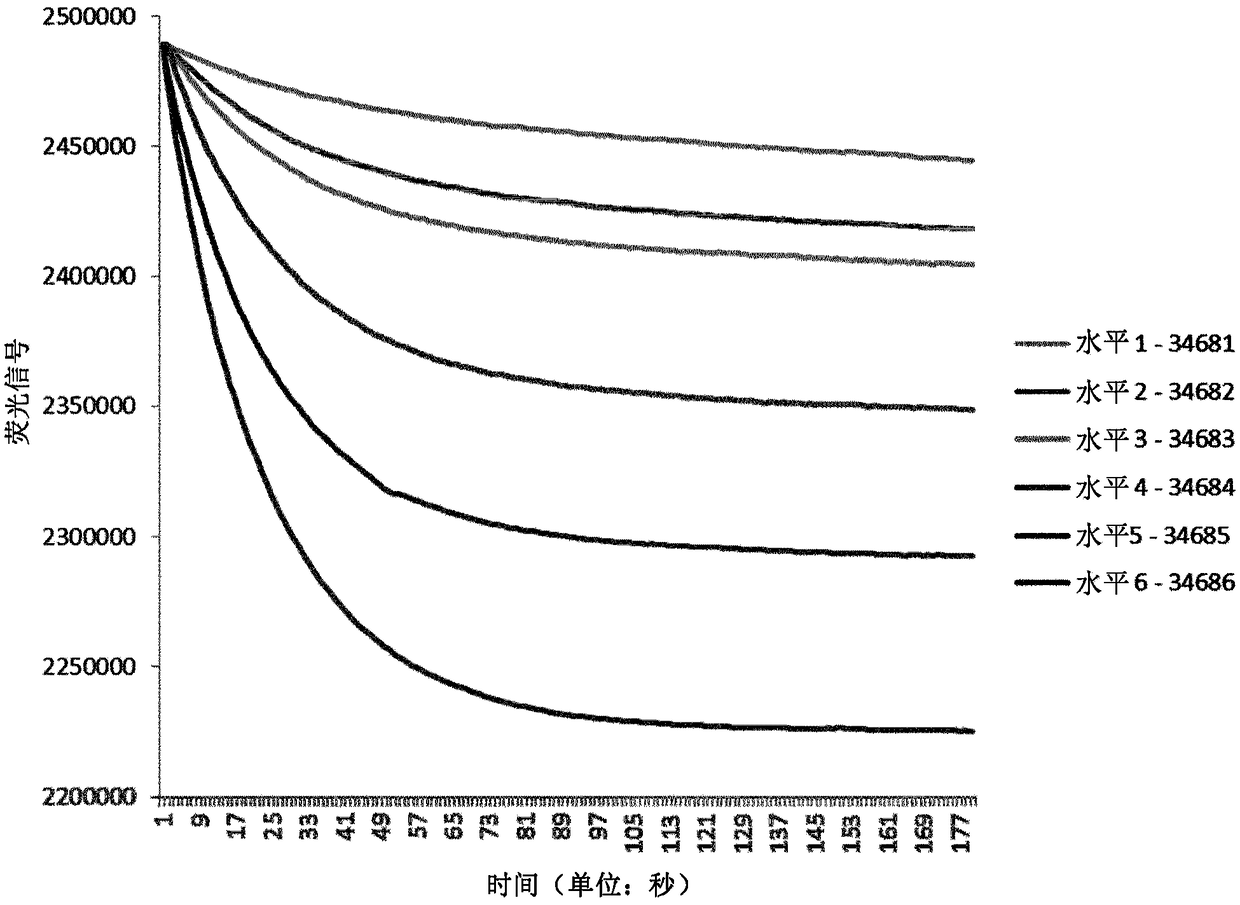
[0099] The six linear samples and raw signal values for TRACE measurements are reported in Table 1 below.
[0100] Table 1
[0101]
[0102] Table 1 shows the TRACE measurements of the six calibrators (BIO-RAD). In column 2, the TRACE fluorescence signal is shown. In column 3, the total hemoglobin signal is shown, while in column 4, the final TRACE HbA1c raw signal is shown. Column 1 shows six different levels of HbA1c, each level representing a specific HbA1c concentration. Column 2 shows the raw signal of HbAlc reported in the 510 nm fluorescence channel 20 seconds after the decrease in fluorescence was measured after the addition...
example 2
[0118] Example 2. HbA1c Accuracy Process
[0119] According to Example 1, a sample of 40 patients obtained from the Herlev Hospital was measured with respect to TRACE. Known values reported in NGSP format were obtained from the Roche cobas c system (Tina-quant HbA1c assay) for each sample.
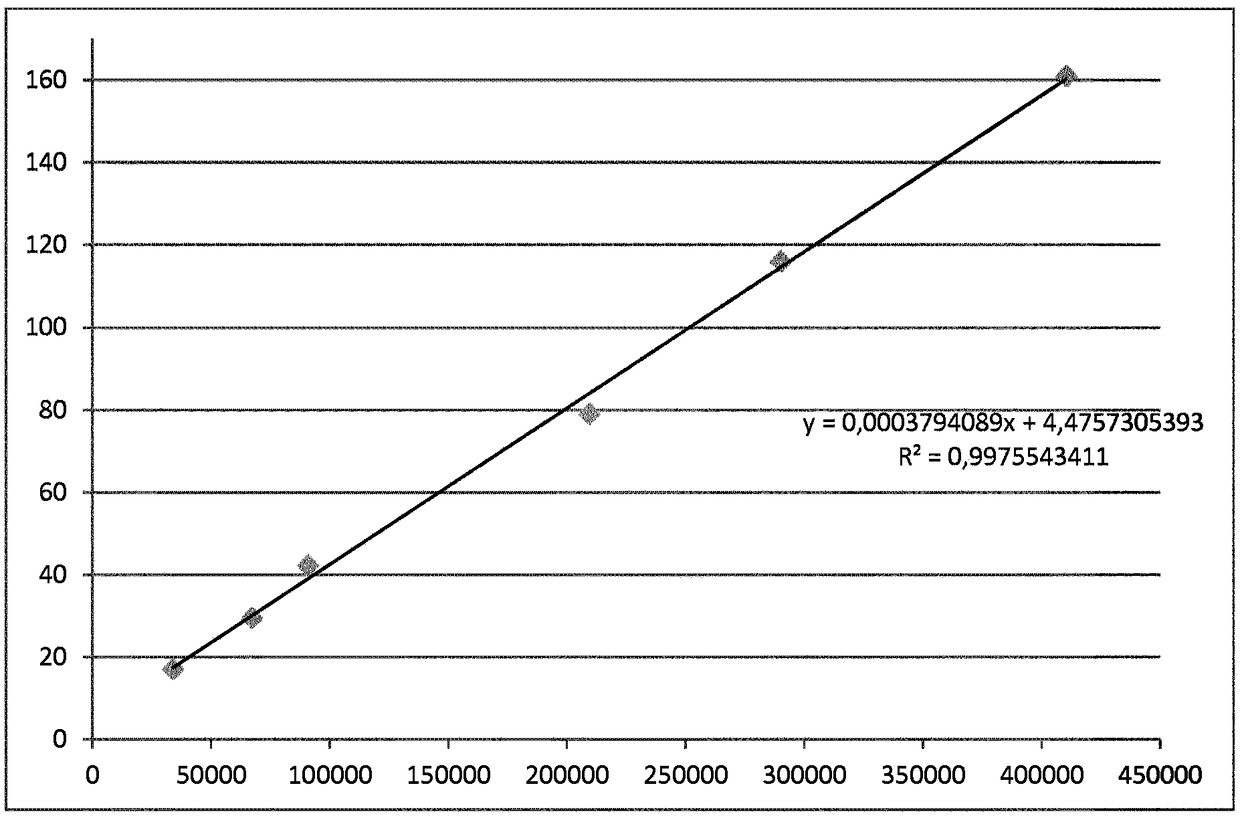
[0120] In Table 3, using Figure 4 The calibration algorithm shown in converts the TRACE raw signal for 40 patient samples into 40 NGSP values.
[0121] table 3
[0122]
[0123] Figure 5 The content of Table 3 is shown graphically, where the raw TRACE HbA1c signal from 40 patients is shown (x-axis) as a function of the final TRACE NGSP signal (y-axis).
[0124] In Table 4, samples from 40 patients run on TRACE are compared to samples from the same 40 patients run on the Roche cobas c system (Tina-quant HbA1c assay format).
[0125] Table 4
[0126]
[0127] Figure 6 The content of Table 4 is shown graphically, however, the TRACE HbA1c NGSP results from 40 patients are sho...
example 3
[0134] Example 3. HbA1c Accuracy Process
[0135] Liquichek diabetes control levels 1, 2 and 3 (BIO-RAD criteria) were measured on TRACE over five days. Each control was measured four times per day. A total of 3×4×5=60 measurements are performed according to examples 1 and 2.
[0136] Calibration was performed to the Tina-quant HbA1c assay format and values were reported in NGSP units.
[0137] Table 6 shows the means as well as the within and between %CV displays.
[0138] Table 6
[0139]
[0140] According to the NGSP plan, the recommendation for the accuracy of the HbA1c assay should be less than 3.5%. As shown in Table 6, the method according to the present invention brings this standard into compliance with the NGSP recommendation for accuracy standards.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com