Method for measuring atosiban acetate related substance
A technology of atosiban acetate and related substances, which is applied in the field of chemical analysis, can solve problems such as allergic reactions, adverse reactions, and no analysis and identification methods, and achieve the effect of controlling related substances and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The present embodiment provides a method for determining related substances of atosiban acetate, comprising the following steps:
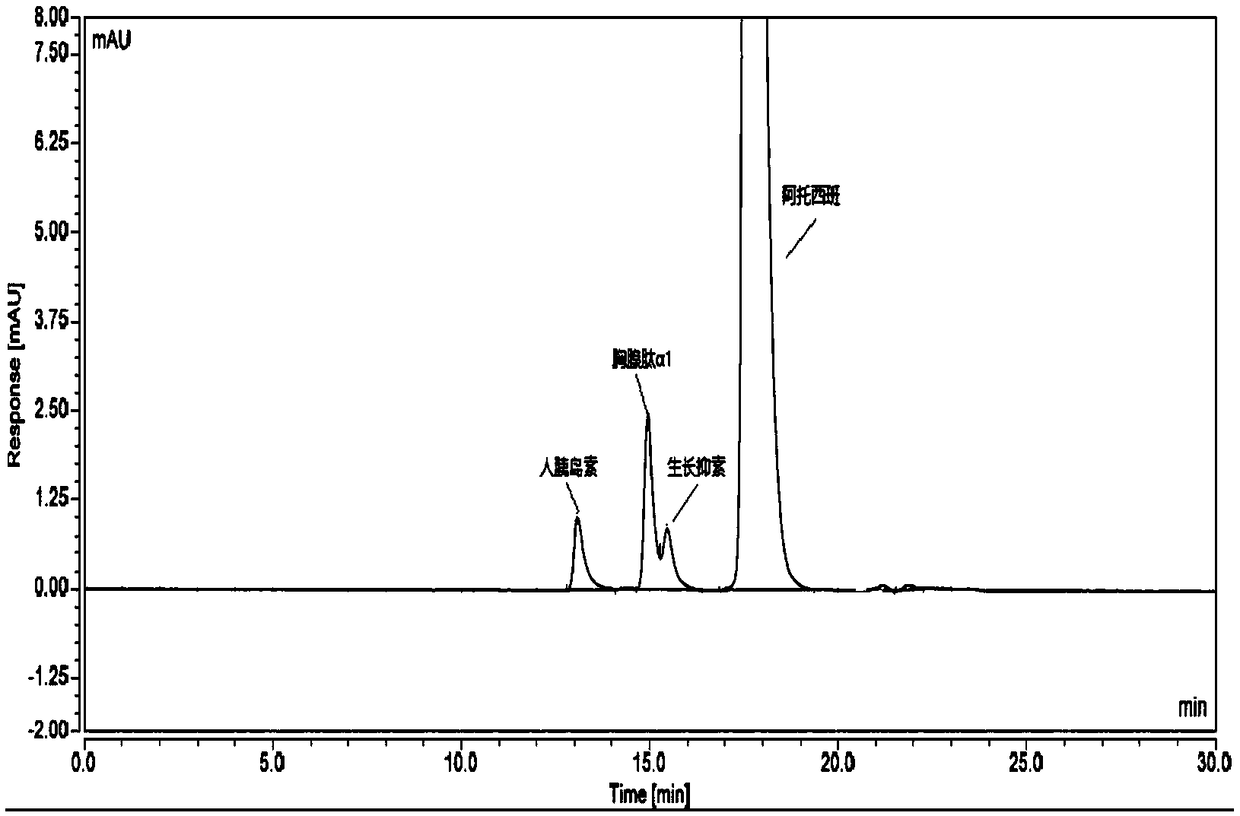
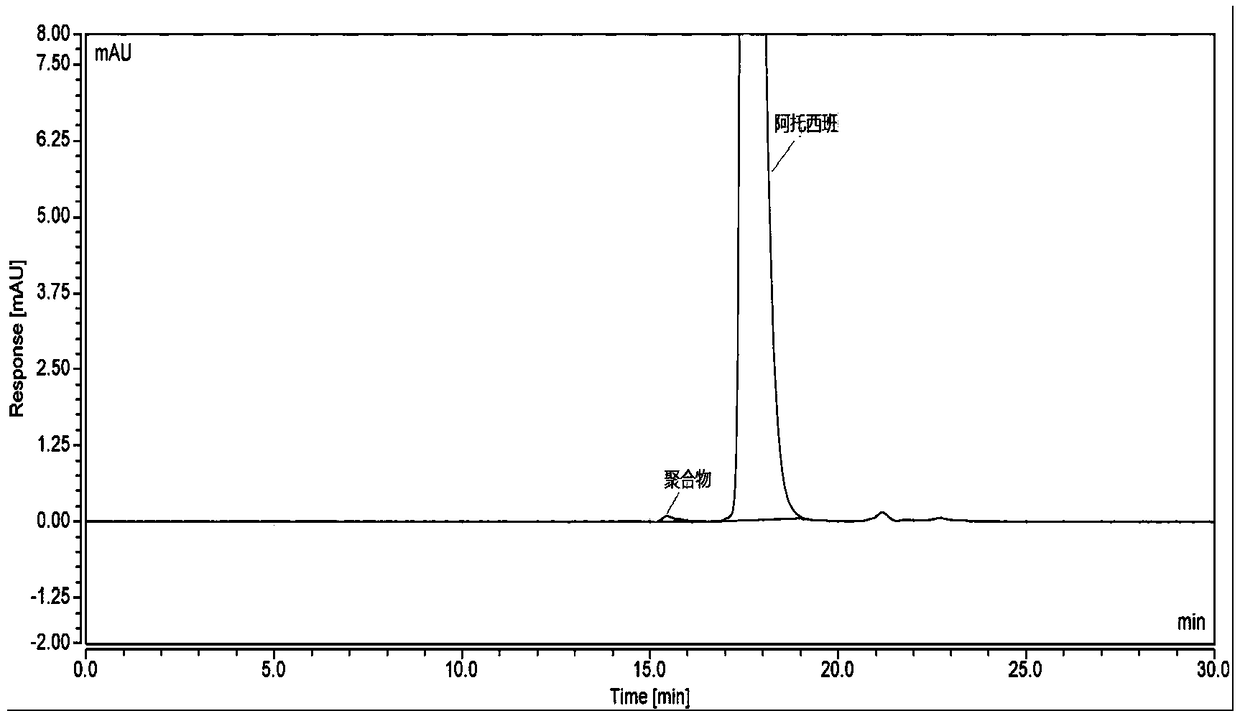
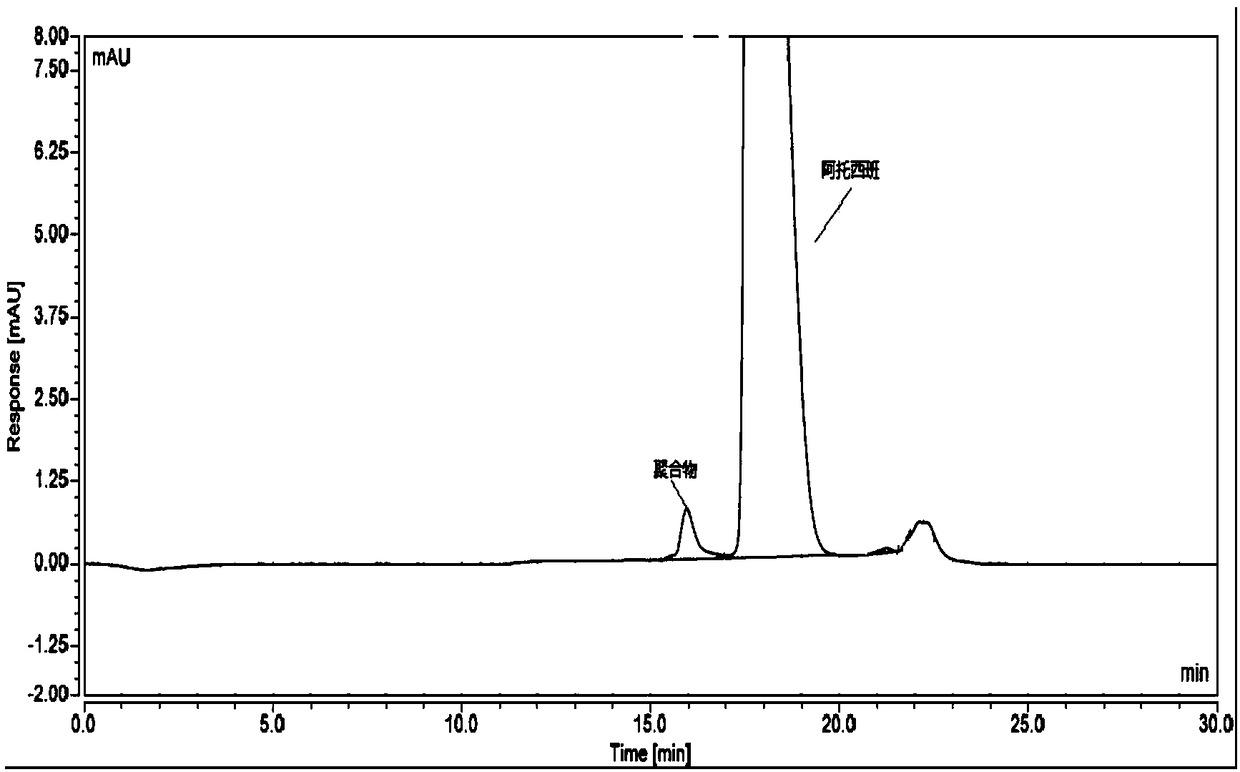
[0049] Take atosiban acetate (the atosiban acetate after the strong degradation experiment has been carried out), add mixed solution to dissolve and dilute to make the solution containing atosiban acetate 1.0mg in every 1ml, as need testing solution; Precisely measure an appropriate amount of the test solution, dilute it with a mixed solution to make a solution containing 10 μg of atosiban acetate per 1 ml, as the sample injection solution. Inject the sample and record the chromatogram.
[0050] Take human insulin (molecular weight 5808) reference substance, thymosin α1 (molecular weight 3108) reference substance, somatostatin (molecular weight 1638) reference substance and atosiban acetate reference substance respectively, add mixed solution to dissolve and dilute to make each 1ml A solution containing 100 μg / ml of human insulin, thymosin ...
Embodiment 2-5
[0056] The method for determining related substances of atosiban acetate provided in Examples 2-5 is basically the same as that in Example 1, except that the operating conditions change.
Embodiment 2
[0057] Embodiment 2: chromatographic conditions: the mass percent of need testing solution is 1.5mg / ml, and the volume ratio of mobile phase A and mobile phase B is 80:20, and mobile phase A is the 1mol / ml phosphate solution that pH is 2.0, The mobile phase B is methanol, the flow rate is 1.1ml / min, the column temperature is 21-37°C; the detection wavelength is 225nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com