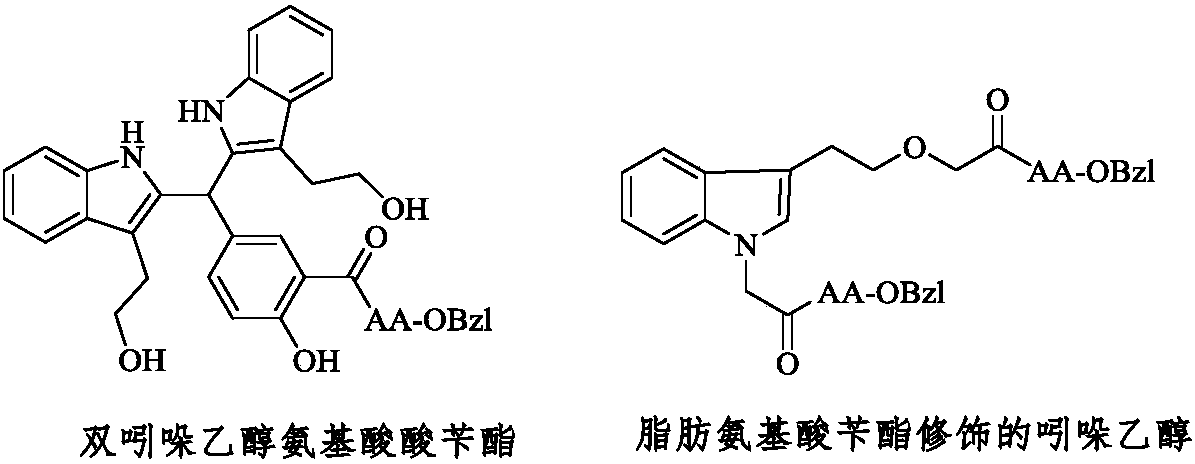
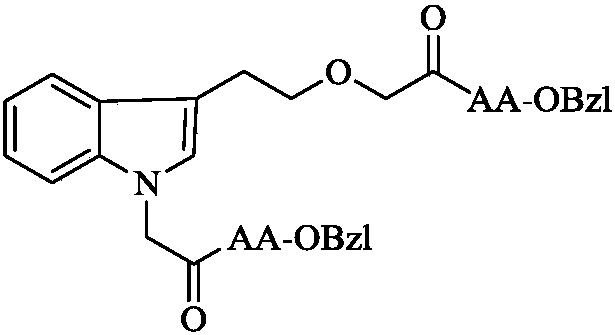
Fatty amino acid modified indole ethanol derivative, synthesis, activities and applications thereof
A technology of indole and ethoxyacetyl, which is used in the preparation of antitumor drugs, 1--3-indole, and the field of anti-inflammatory activity, and can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
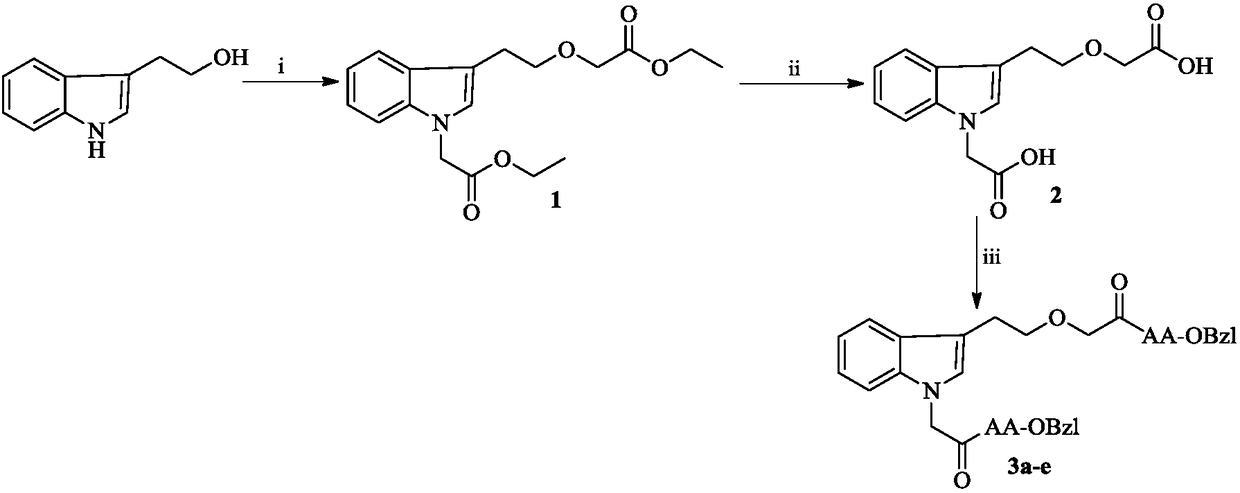
[0015] Example 1 Preparation of 1-ethoxycarbonylmethyl-3-ethoxycarbonylmethoxyethyl-indole (1)
[0016] Slowly add 2.98g (4mmol, 60%) NaH to a solution of 5.00g (31mmol) indole alcohol and 50mL anhydrous tetrahydrofuran (THF) at room temperature, and slowly add 17.22mL (5mmol) ethyl bromoacetate after stirring for 30 minutes , heated at 80°C for 48 hours. TLC (petroleum ether / ethyl acetate, 3 / 1) showed the reaction was complete. Heating was stopped and the reaction mixture was cooled to room temperature. The solid was filtered off, and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate, 3 / 1) to obtain 1.54 g (15%) of the title compound as a yellow syrup. ESI-MS(m / e):334[M+H] + .
Embodiment 2
[0017] Embodiment 2 prepares 1-carboxymethyl-3-carboxymethoxy ethyl indole (2)
[0018] 1.31 g (3.9 mmol) of 1-ethoxycarbonylmethyl-3-ethoxycarbonylmethoxyethyl-indole (1) was dissolved in 10 mL of methanol under ice cooling. A 2N NaOH aqueous solution was added dropwise to the obtained solution to adjust the pH of the solution to 12. After stirring for 5 hours, TLC (petroleum ether / ethyl acetate, 3 / 1) showed that the reaction was complete. The reaction mixture was saturated with KHSO 4 The pH of the aqueous solution was adjusted to 7, concentrated under reduced pressure, the residue was extracted 3 times with 15 mL ethyl acetate, and the aqueous layer was washed with saturated KHSO 4 The aqueous solution was adjusted to pH 4 and extracted 3 times with 15 mL of ethyl acetate. The separated aqueous layer continued to be saturated with KHSO 4 Adjust the pH of the aqueous solution to 2, extract 3 times with 15 mL ethyl acetate and combine the separated ethyl acetate layers, wa...
Embodiment 3
[0019] Example 3 Preparation of 1-(acetyl-Ala-OBzl)-3-(ethoxyacetyl-Ala-OBzl) indole (3a)
[0020] A solution of 0.55 g (2.0 mmol) of 1-carboxymethyl-3-carboxymethoxyethyl-indole (2), 0.54 g (4.0 mmol) of N-hydroxybenzotriazole and 10 mL of anhydrous THF was stirred After 30 minutes, reaction solution A was obtained. 0.82 g (4.0 mmol) of dicyclohexylcarbodiimide was dissolved in 5 mL of anhydrous THF to obtain a reaction solution B. Under ice-cooling, the reaction solution B was slowly added dropwise to the reaction solution A, and stirred for 30 minutes. Then, a solution of 1.72 g (8.0 mmol) of HCl·Ala-OBzl and 15 mL of anhydrous THF was added thereto. The reaction mixture was adjusted to pH 9 with N-methylmorpholine and stirred at room temperature for 10 hours. TLC (CH 2 Cl 2 / CH 3 OH, 30 / 1, plus 3 drops of acetic acid) showed completion of the reaction. The reaction mixture was filtered, the filtrate was concentrated under reduced pressure, and the residue was dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com