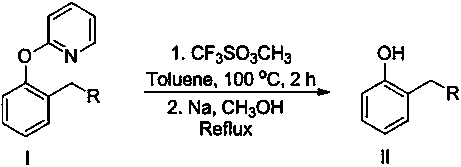Preparation method of ortho-alkylphenol
A technology of ortho-alkylphenol and alkylphenoxy, which is applied in the field of chemistry, can solve the problems affecting the synthesis efficiency of ortho-alkylaniline compounds, waste of raw materials, etc., and achieves an easy-to-implement, simple route and few side reactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
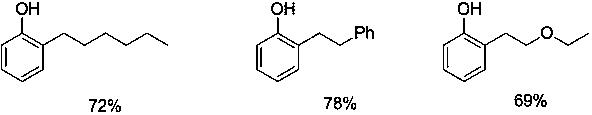
[0011] Add 2-(2--hexyl)phenoxy)pyridine 255mg (1 mmol), methyl trifluoromethanesulfonate 328mg (2mmol), toluene 10 mL in the reaction tube, under nitrogen, 100 o C stirred for 24 hours, after the reaction, the crude product was separated, then added to the reaction tube, added sodium metal (15mmol) and methanol (15mL), refluxed for 15 minutes, cooled, and separated by column chromatography to obtain 128mg of the target product 2-hexylphenol, The yield was 72%.
Embodiment 2
[0013] Add 275 mg (1 mmol) of 2-(2-phenethylphenoxy)pyridine, 328 mg (2 mmol) of methyl trifluoromethanesulfonate, and 10 mL of toluene to the reaction tube. Under nitrogen, 100 o C stirred for 24 hours. After the reaction, the crude product was separated, then added to the reaction tube, added sodium metal (15mmol) and methanol (15mL), refluxed for 15 minutes, cooled, and separated by column chromatography to obtain the target product 2-phenethylphenol 154 mg, yield 78%.
Embodiment 3
[0015] Add 2-(2-(ethoxyethyl)phenoxy)pyridine 243mg (1 mmol), methyl trifluoromethanesulfonate 328mg (2 mmol) and toluene 10 mL in the reaction tube, under nitrogen, 100 o C stirred for 24 hours, after the reaction, the crude product was separated, then added to the reaction tube, added sodium metal (15mmol) and methanol (15mL), refluxed for 15 minutes, cooled, and separated by column chromatography to obtain the target product 2-ethoxyethyl Base phenol 115mg, productive rate is 69%.
[0016] The following are the synthetic products and corresponding yields using the technical scheme of the present invention.
[0017]
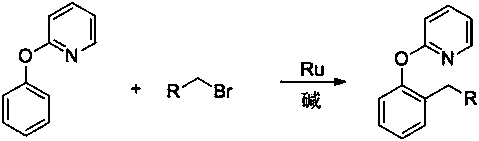
[0018] In the present invention, 2-(2-alkylphenoxy)pyridine derivatives are prepared by using 2-phenoxypyridine derivatives and bromoalkane, which is a primary bromoalkane, and the reaction formula is as follows:
[0019]
[0020] Where: R is an alkyl group or an aryl group or an ether group, and 2-phenoxypyridine derivatives, primary brominated alkanes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com