Heteropolycyle compound having photo-activity and preparation method thereof
A compound and heteropolycyclic technology, applied in the field of photoactive heteropolycyclic compounds and their preparation, can solve the problems of excess low-end products, insufficient application research and development, backward technical level, etc., and achieve convenient sources, low cost, and environmental protection. Friendly and pollution-free effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
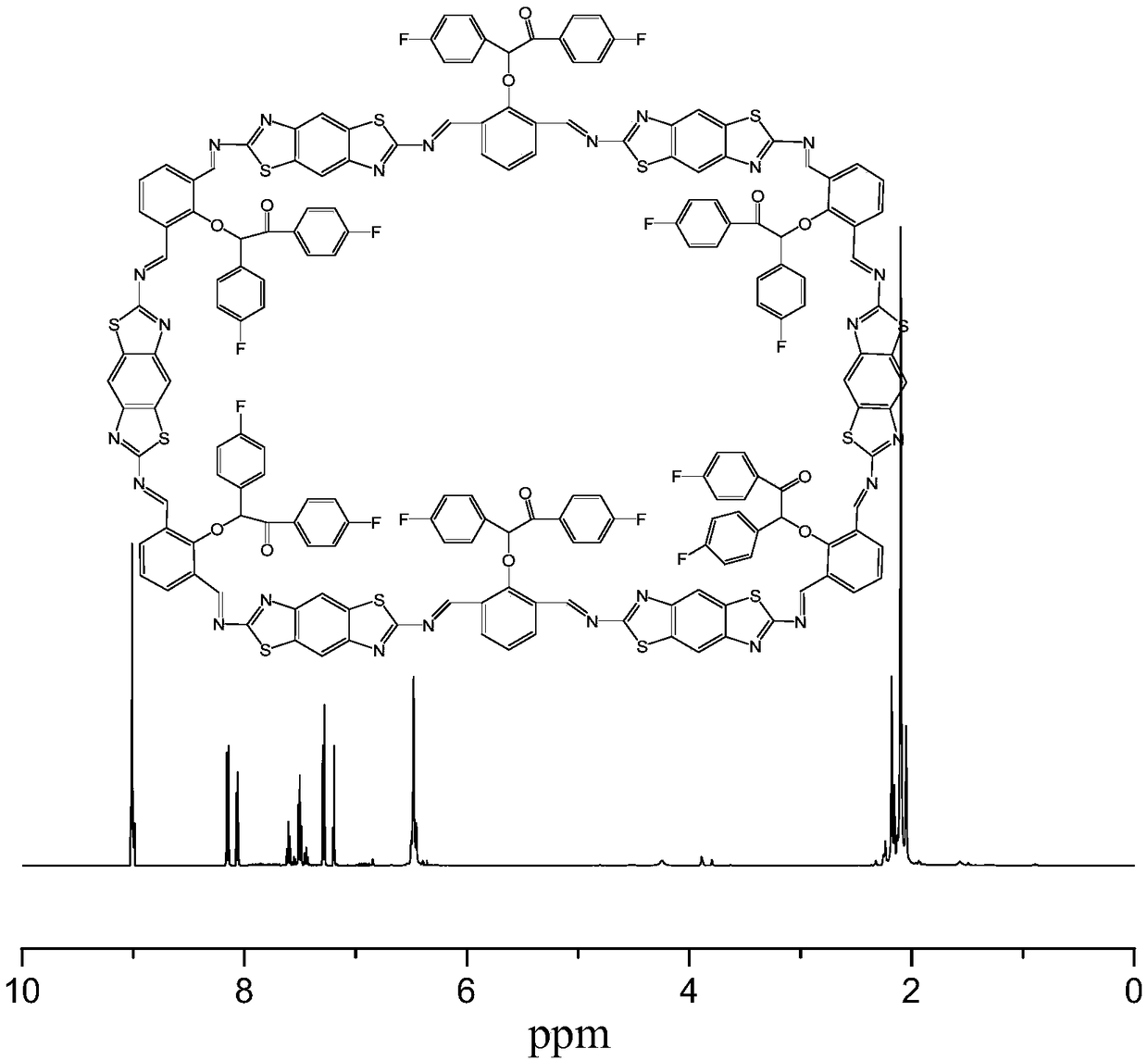
[0038] 1. Preparation of photoactive heteropolycyclic compounds
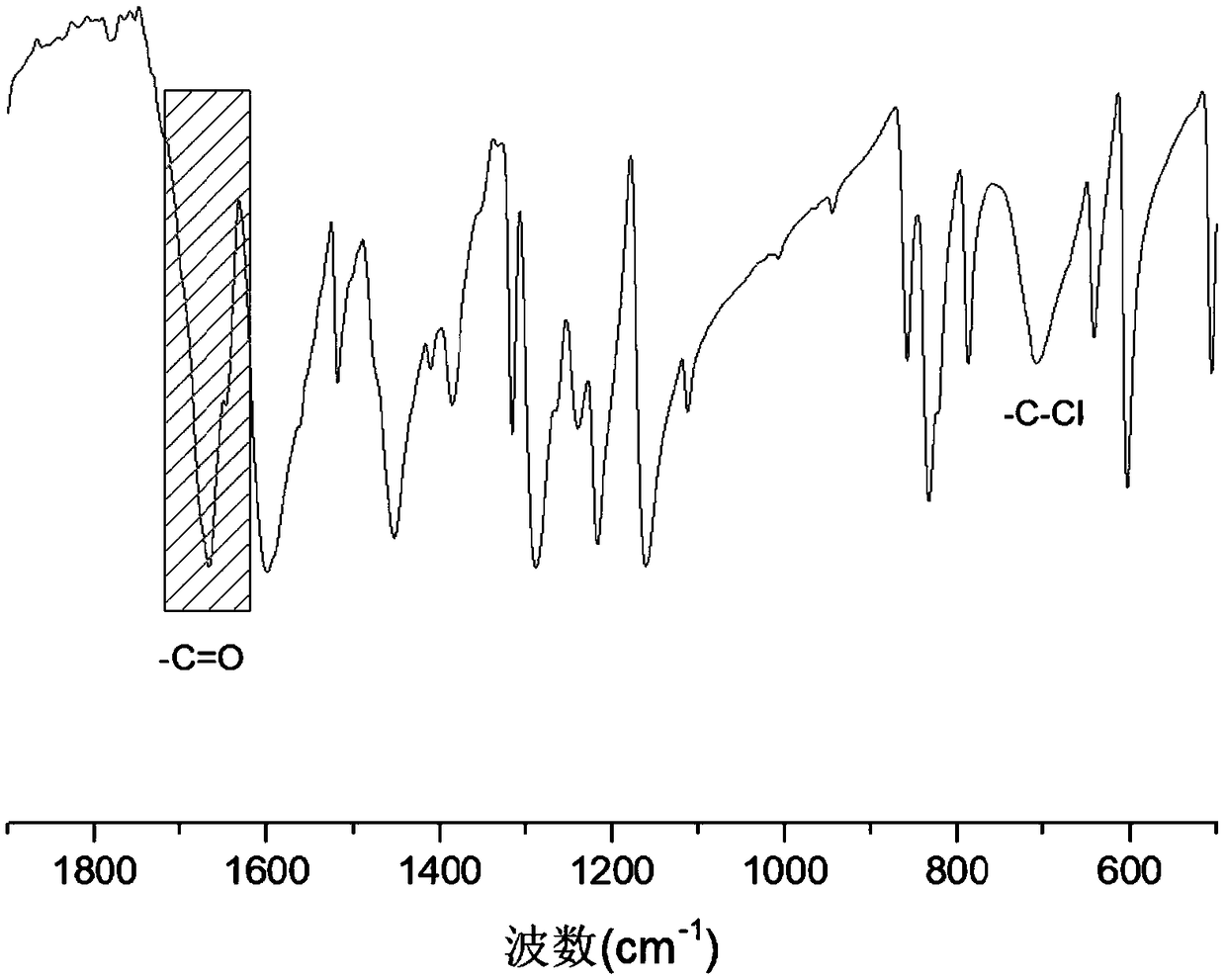
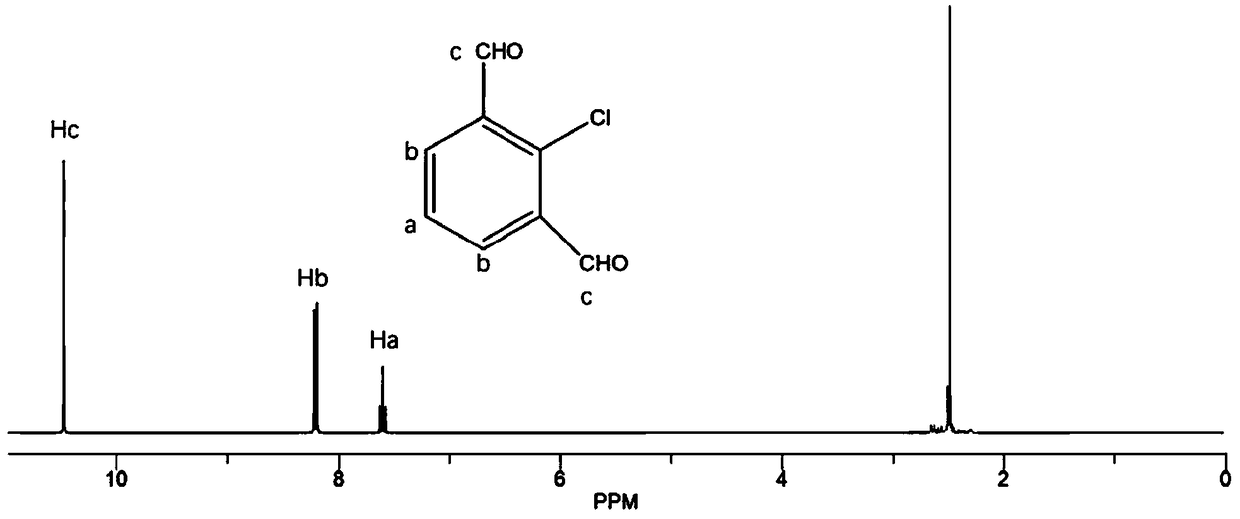
[0039] (1) 2-chloroisophthalaldehyde and 2,6-diaminobenzodithiazole with a molar ratio of 1:1.1 are added to the reaction vessel, a single solvent mesitylene is added dropwise, the volume of mesitylene and the reaction The volume-to-weight ratio of the total mass of the reactant is 10 milliliters: 1 gram, then add 0.5% acetic acid of the total mass of the reactant, and perform ultrasonic treatment to make it mix uniformly;
[0040] (2) Place the reaction vessel in liquid nitrogen to freeze, vacuumize, seal the opening of the reaction vessel, and react for 3 days at a reaction temperature of 110° C.;
[0041] (3) After the reaction, the solvent was distilled off under reduced pressure, dissolved in dichloromethane, washed 3 times with deionized water, dried overnight with anhydrous sodium sulfate, filtered, rotary evaporated to remove dichloromethane, and vacuum-dried to obtain intermediate product;
[0042] (4...
Embodiment 2
[0049] 1. Preparation of photoactive heteropolycyclic compounds
[0050] (1) 2-chloroisophthalaldehyde and 2,6-diaminobenzobithiazole are added into the reaction vessel with a molar ratio of 1:1.3, and a single solvent 1,4-dioxane is added dropwise, 1, The volume-to-weight ratio of the volume of 4-dioxane to the total mass of the reactant is 8 milliliters: 1 gram, then add hydrochloric acid with 0.8% of the total mass of the reactant, and ultrasonically treat it to make it evenly mixed;
[0051] (2) Place the reaction vessel in liquid nitrogen to freeze, vacuumize, seal the opening of the reaction vessel, and react for 4 days at a reaction temperature of 90° C.;
[0052] (3) After the reaction, the solvent was distilled off under reduced pressure, dissolved in dichloromethane, washed 3 times with deionized water, dried overnight with anhydrous sodium sulfate, filtered, rotary evaporated to remove dichloromethane, and vacuum-dried to obtain intermediate product;
[0053] (4) ...
Embodiment 3
[0060] 1. Preparation of photoactive heteropolycyclic compounds
[0061] (1) 2-chloroisophthalaldehyde and 2,6-diaminobenzodithiazole with a molar ratio of 1:1.5 are added to the reaction vessel, and the volume of mesitylene and 1,4-dioxane is added dropwise A mixed solvent with a ratio of 1:3, the volume-to-weight ratio of the volume of the mixed solvent to the total mass of the reactants is 15 milliliters: 1 gram, then add sulfuric acid with 1% of the total mass of the reactants, and perform ultrasonic treatment to make it evenly mixed;
[0062] (2) Place the reaction vessel in liquid nitrogen to freeze, vacuumize, seal the opening of the reaction vessel, and react for 2 days at a reaction temperature of 135° C.;
[0063] (3) After the reaction, the solvent was distilled off under reduced pressure, dissolved in dichloromethane, washed 3 times with deionized water, dried overnight with anhydrous sodium sulfate, filtered, rotary evaporated to remove dichloromethane, and vacuum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com