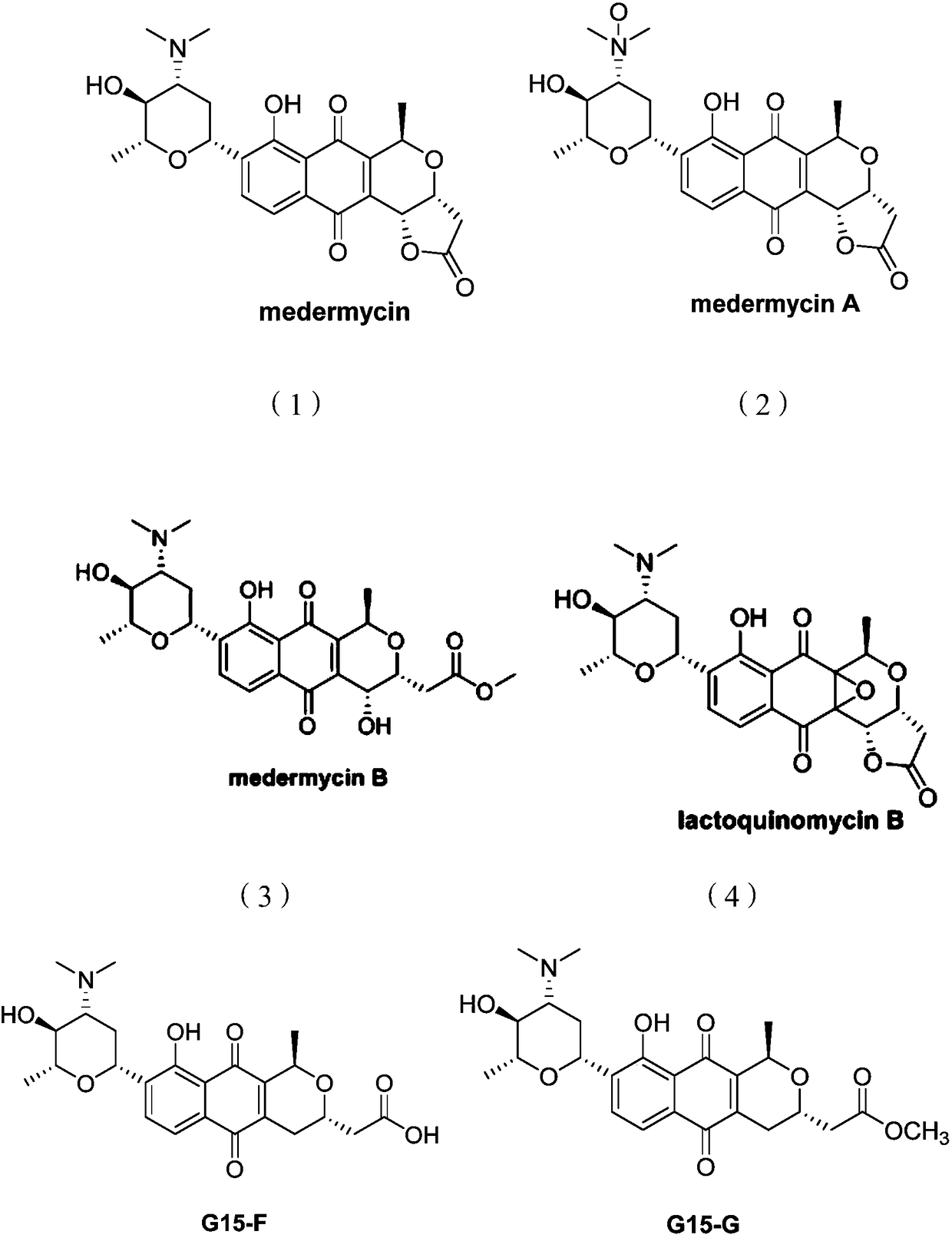
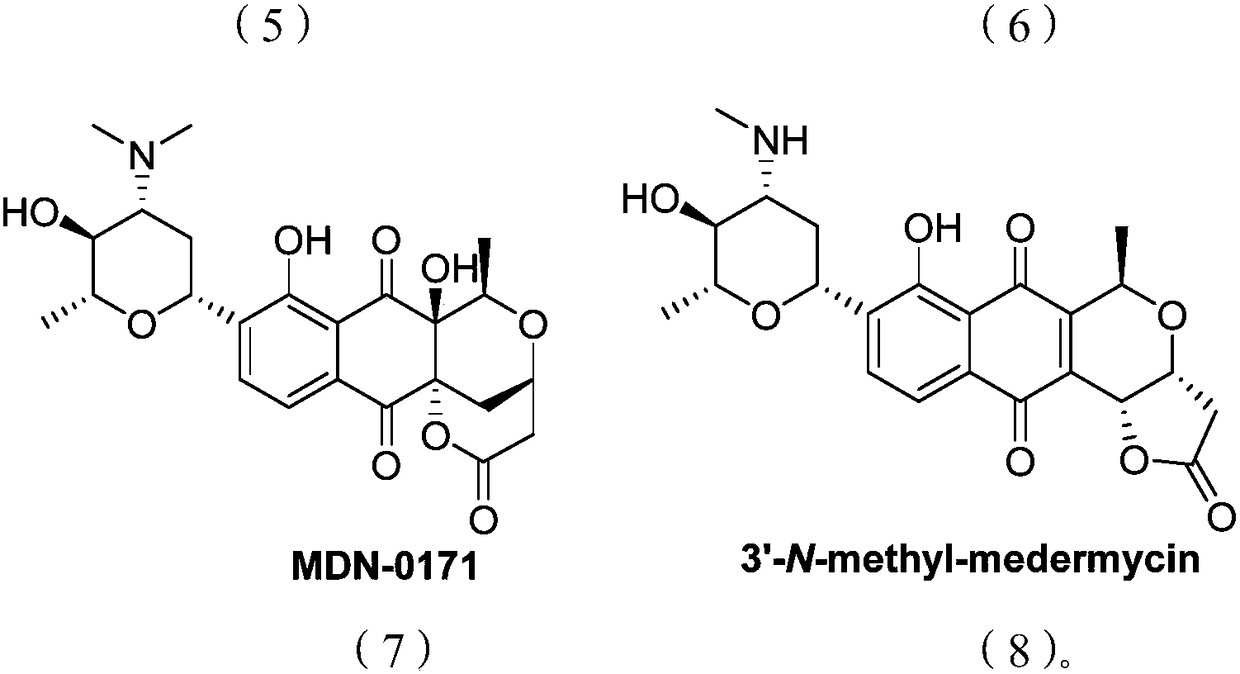
Medermycin compounds as well as preparation method and application thereof
A technology for metamycin and its compounds, which is applied in the field of metamycin compounds and their preparation, can solve the problems of high toxicity, no specificity of metamycin, and the inability to make medicines, etc., and achieve good kinase inhibition and inhibition The effect of growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1. Fermentation of compounds
[0053] 1) Take an appropriate amount of actinomycetes from the original storage slant or glycerol tube, inoculate it on the solid medium of Gaoshi No. 1 plate, let it stand in an incubator at 28°C, and activate it for 4 days.
[0054] 2) Inoculate the single colony of actinomycetes activated in step 1) into a 500mL Erlenmeyer flask containing 250mL liquid medium, each bottle containing 250mL Gaoshi No. 1 liquid medium, in a shaker at 28°C Shake culture at 180rpm for 8 days to obtain fermentation broth.
[0055] 2. Preparation of compounds
[0056] (a) The fermentation broth was extracted three times with an equal volume of ethyl acetate, and the obtained extract was dried in vacuo to remove the ethyl acetate solvent. The obtained ethyl acetate fraction was subjected to silica gel column chromatography, and the dichloromethane-methanol system with a volume ratio of 100:1 to 0:1 was used for gradient elution. Fractions containing new compo...
Embodiment 2
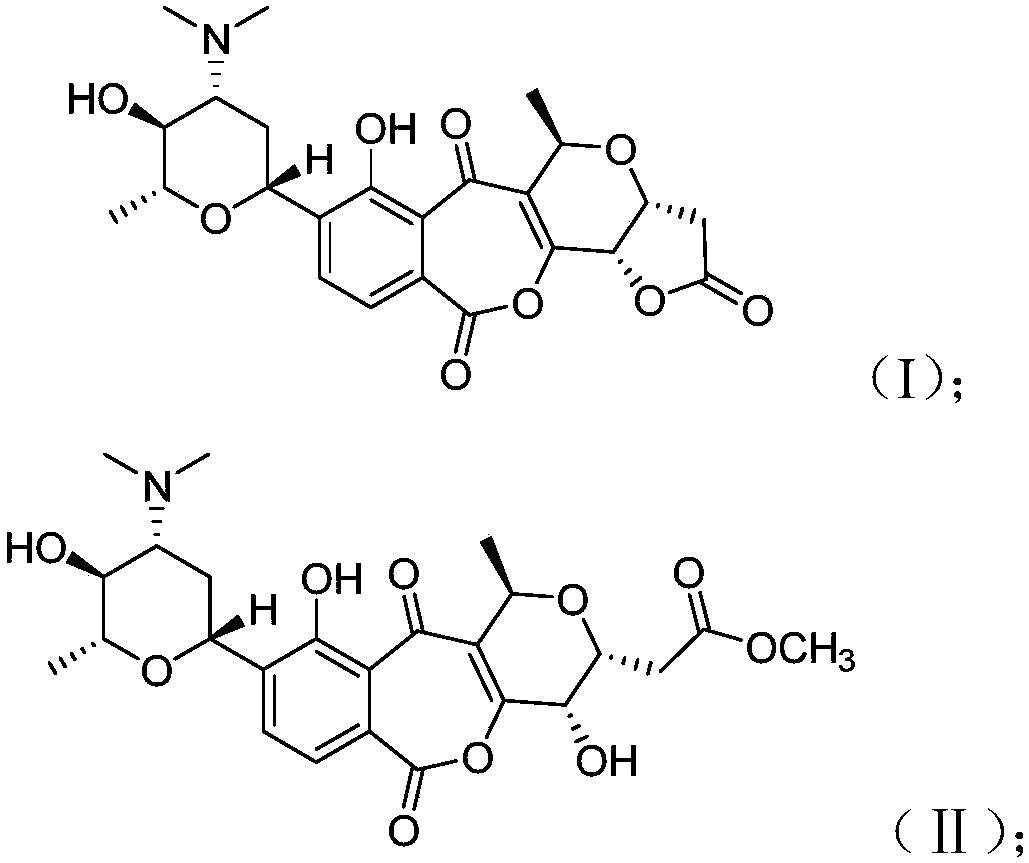
[0065] The obtaining of fermented liquid is the same as in Example 1, the only difference is that in the preparation step (c) of the compound, the fractions containing the new compound are separated by reverse phase high performance liquid chromatography (Agilent Pursuit C-18 (10 μm, 21.2 × 250mm ) chromatographic column, the detection wavelength is 230nm, the mobile phase used is a methanol-water system with a methanol volume ratio of 10 to 100% containing 0.05% trifluoroacetic acid, and is eluted with a gradient of 20mL / min for 40 minutes, and the elution of 10 to 12 minutes is collected. The structure of the compound obtained by separation and purification was identified, and the molecular formula was calculated as C 25 h 32 NO 10 ([M+H] + m / z 506.2020), further based on nuclear magnetic resonance data, its specific structure is as follows, denoted as strepoxepinmycin B:
[0066]
[0067] The NMR data for this compound are listed in Table 2 below.
[0068] Table 2
...
Embodiment 3
[0072] The obtaining of fermented liquid is the same as in Example 1, the only difference is that in the preparation step (c) of the compound, the fractions containing the new compound are separated by reverse phase high performance liquid chromatography (Agilent Pursuit C-18 (10 μm, 21.2 × 250mm ) chromatographic column, the detection wavelength is 254nm, the mobile phase used is a methanol-water system with a methanol volume ratio of 10 to 100% containing 0.05% trifluoroacetic acid, and is eluted with a gradient of 20mL / min for 40 minutes, and the elution of 15 to 17 minutes is collected. The structure of the compound obtained by separation and purification was identified, and the molecular formula was calculated as C 25 h 34 NO 10 ([M+H] + m / z 508.2179), further based on nuclear magnetic resonance data, its specific structure is as follows, denoted as strepoxepinmycin C:
[0073]
[0074] The NMR data for this compound are listed in Table 3 below.
[0075] table 3
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com