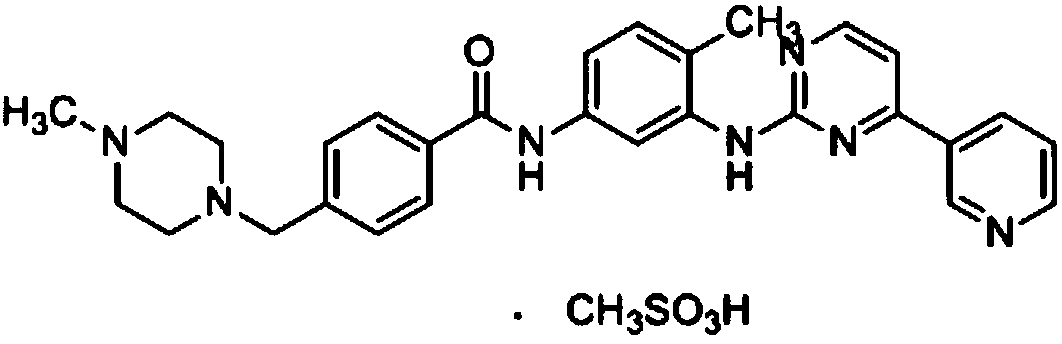
Compound containing imatinib and plant polysaccharide
A technology of plant polysaccharide and composition, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
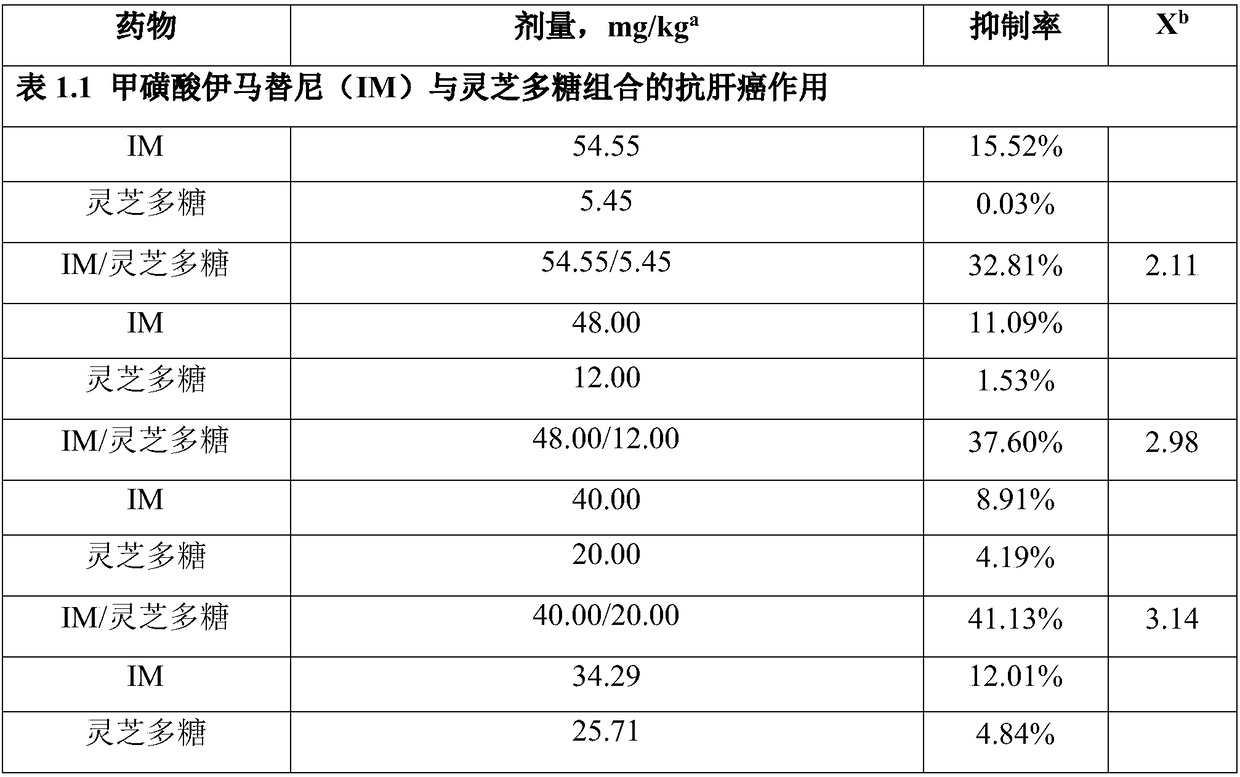
[0073] Example 1 Preparation of capsules comprising imatinib mesylate and ganoderma polysaccharide
[0074] Prescription (according to 100 capsules)
[0075]
[0076] Preparation
[0077] Get the prescribed amount of imatinib mesylate, ganoderma polysaccharide, microcrystalline cellulose and sodium stearyl fumarate, mix thoroughly, fill capsules, and pack separately to obtain 100 capsules.
Embodiment 2
[0078] Embodiment 2 The preparation of the capsule comprising imatinib mesylate and Yunzhi polysaccharide
[0079] Prescription (according to 100 tablets)
[0080]
[0081]
[0082] Preparation
[0083] Take the prescribed amount of imatinib mesylate, versicolor polysaccharide, microcrystalline cellulose and sodium stearyl fumarate, fully mix, and press into 100 tablets.
Embodiment 3
[0084] Example 3 Preparation of Capsules Comprising Imatinib Mesylate and Astragalus Polysaccharide
[0085] prescription
[0086]
[0087] Preparation
[0088] Take the prescribed amount of imatinib mesylate, astragalus polysaccharide, microcrystalline cellulose and sodium stearyl fumarate, mix them thoroughly, and pack them separately.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com