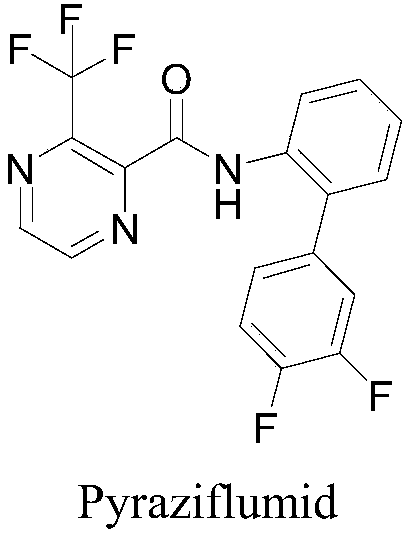
Preparation method and application of 6-chloropyrazine-2-amide compounds containing diphenyl ether groups
A technology of amide compounds and chloropyrazine, which is applied in the field of preparation and application of 6-chloropyrazine-2-amide compounds containing diphenyl ether groups, and can solve the problems of no research report on herbicidal activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
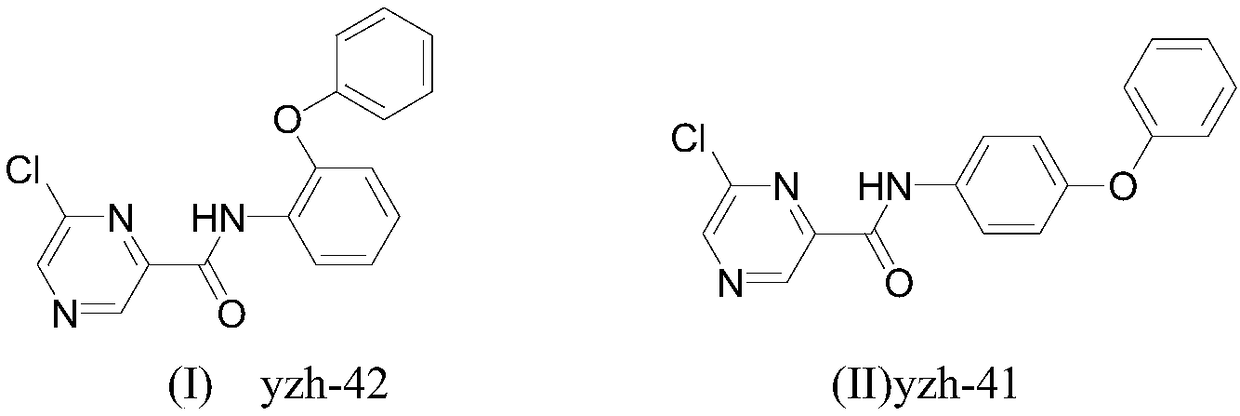
[0026] Preparation of 6-chloro-N-(2-phenoxyphenyl)pyrazine-2-carboxamide:
[0027] Add 2-phenoxyaniline (0.185g, 1.0mmol), 6-chloropyrazine-2-carboxylic acid (0.158g, 1.0mmol) and 30mL dichloromethane in the reaction flask, add Et 3 N (0.202g, 2.0mmol), then added EDCI (0.287mg, 1.5mmol), HOBt (0.20g, 1.5mmol), reacted at 25°C for 2.5 hours, TLC detected that the reaction was complete, the reaction was complete, and the reaction solution was washed twice with water , washed once with saturated brine, dried the organic phase with anhydrous sodium sulfate, precipitated to obtain a crude product, recrystallized from ethanol to obtain a brick red solid, m.p.138-140°C, yield 79.0%, 1 H-NMR (400MHz, CDCl 3 )δ: 9.62(s,1H,NH), 9.58(s,1H,pyrazine), 8.83(s,1H,pyrazine), 8.54(s,1H,C 6 h 4 ), 7.82 (d, J=8.4Hz, 2H, C 6 h 4 ), 7.33–7.42 (m,2H,C 6 h 4 ), 7.00–7.15 (m,4H,C 6 h 5 ).HRMS(EI)(calcd.m / z): 325.0612(325.0618,M + ).
Embodiment 2
[0029] Preparation of 6-chloro-N-(4-phenoxyphenyl)pyrazine-2-carboxamide:
[0030] Add 4-phenoxyaniline (0.185g, 1.0mmol), 6-chloropyrazine-2-carboxylic acid (0.158g, 1.0mmol) and 30mL dichloromethane in the reaction flask, add Et 3 N (0.202g, 2.0mmol), then added EDCI (0.287mg, 1.5mmol), HOBt (0.20g, 1.5mmol), reacted at 25°C for 2.0 hours, TLC detected that the reaction was complete, and the reaction was completed, and the reaction solution was washed twice with water , washed once with saturated brine, dried the organic phase with anhydrous sodium sulfate, and desolvated to obtain a crude product, which was recrystallized from ethanol to obtain a white solid, m.p.160-162°C, yield 83.0%, 1 H-NMR (400MHz, CDCl 3 )δ: 9.66(s,1H,NH), 9.53(s,1H,pyrazine), 8.82(s,1H,pyrazine), 8.61(s,1H,C 6 h 4 ), 7.74 (d, J=8.4Hz, 2H, C 6 h 4 ), 7.31–7.39 (m,2H,C 6 h 4 ), 6.95–7.13 (m, 4H, C 6 h 5 ).HRMS(EI)(calcd.m / z): 325.0610(325.0618,M + ).
Embodiment 3
[0032] Determination of Herbicidal Activity of 6-Chloropyrazine-2-amide Compounds Containing Diphenyl Ether Group
[0033] Test design
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com