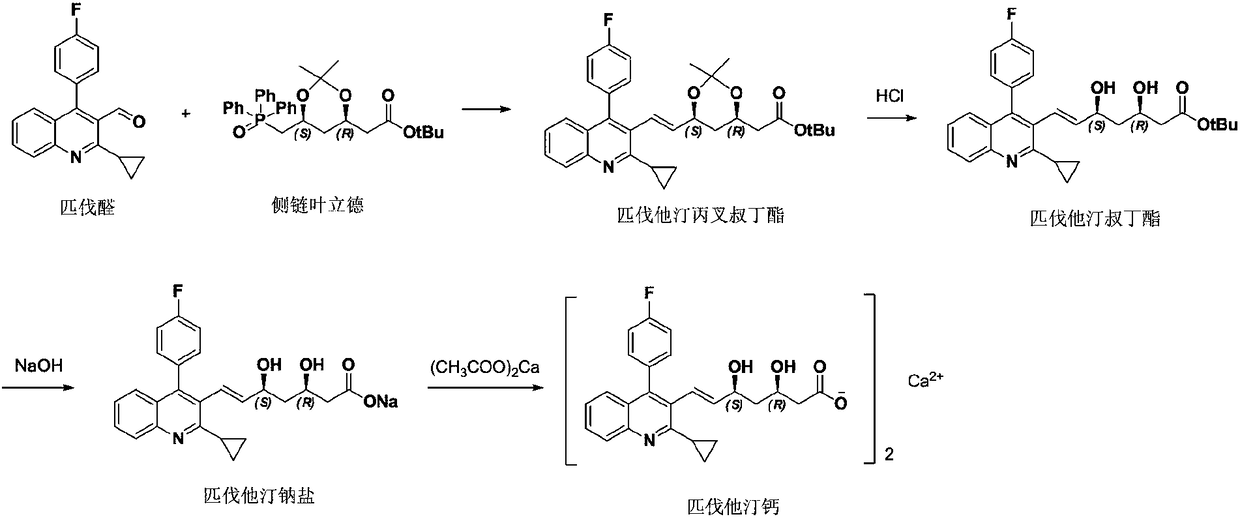
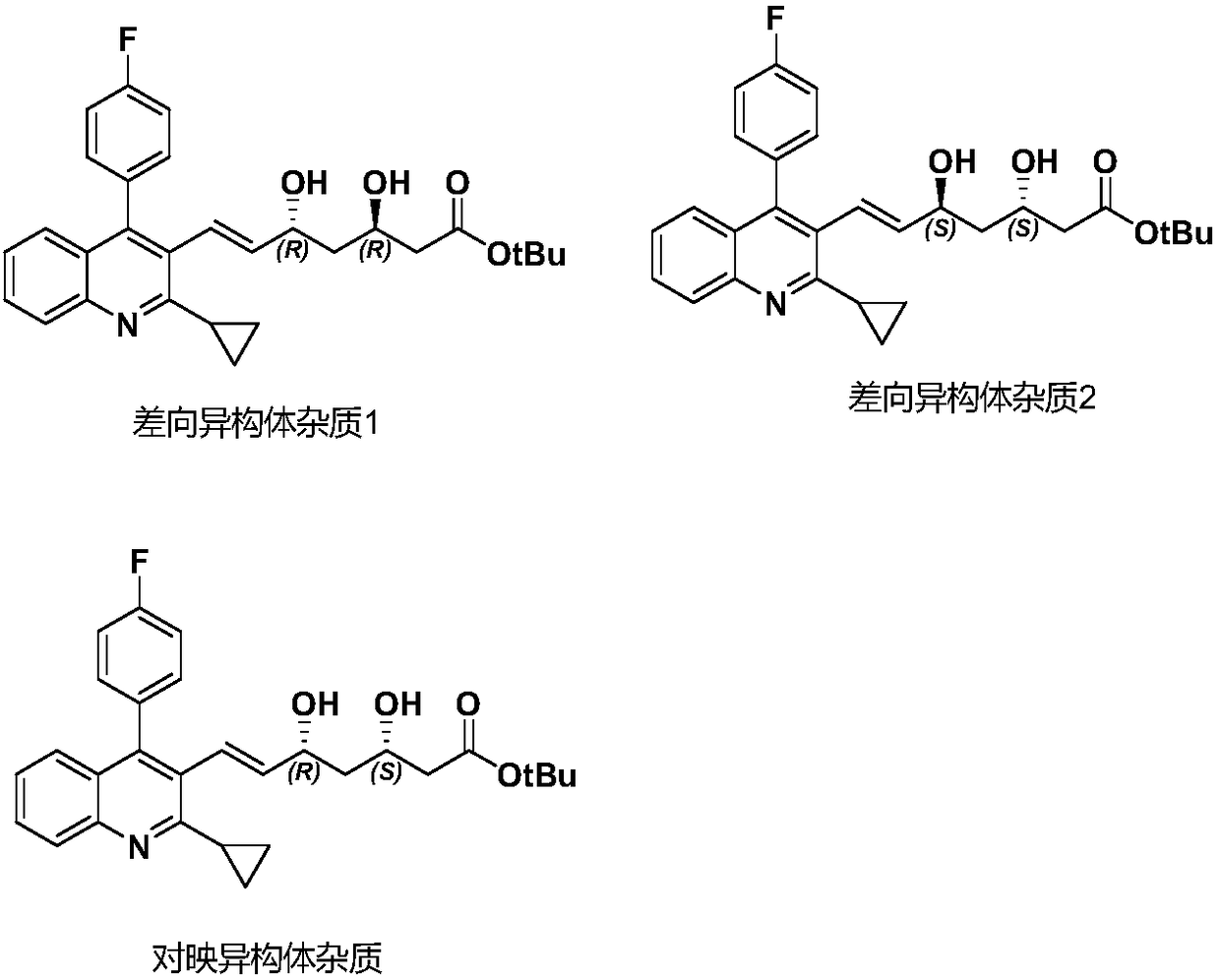
Refining method of pitavastatin tert-butyl ester
A technology of pitavastatin tert-butyl ester and refining method, which is applied in the direction of organic chemistry, organic chemistry, etc., can solve the problems of inability to remove and affect, and achieve the effect of low uncontrollability, good refining effect and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Mix 40g of pitavastatin tert-butyl ester and 120mL of methyl tert-butyl ether, raise the temperature to reflux temperature, stir for 30min, dissolve and clear, add dropwise 360mL of n-heptane, the solution is clear, cool down to 25°C for crystallization for 2h, and filter with suction. The filter cake was vacuum-dried at 50°C for 5 hours to obtain 33.7 g of pitavastatin tert-butyl ester. See Table 2 for the HPLC purity and yield.
Embodiment 2
[0035] Mix 40g of pitavastatin tert-butyl ester, 120mL of methyl tert-butyl ether and 480mL of n-heptane, raise the temperature to reflux temperature, stir for 30min, the solution is clear, cool down to 25°C for crystallization for 2h, suction filter, and vacuum the filter cake at 50°C After drying for 5 hours, 36.5 g of pitavastatin tert-butyl ester was obtained. See Table 2 for HPLC purity and yield.
Embodiment 3
[0037] Mix 40g of pitavastatin tert-butyl ester and 120mL of methyl tert-butyl ether, raise the temperature to reflux temperature, stir for 30min, dissolve, add 600mL of n-heptane dropwise, the solution is clarified, add 0.8g of activated carbon, reflux for 45min to decolorize, while hot After suction filtration, the filtrate was cooled to 25°C to crystallize for 2 hours, then suction filtered, and the filter cake was vacuum-dried at 50°C for 5 hours to obtain 37.1 g of pitavastatin tert-butyl ester. See Table 2 for HPLC purity and yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com