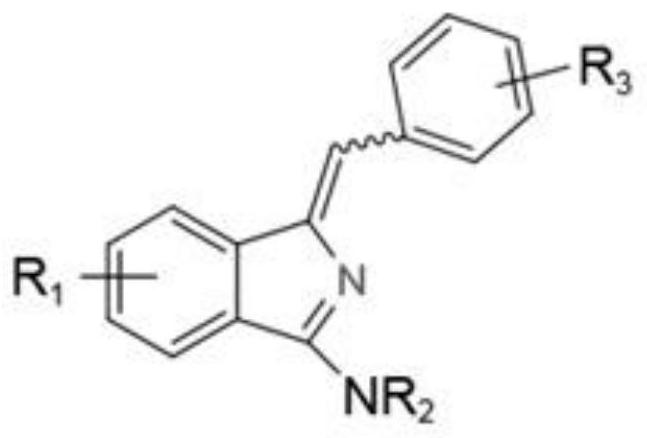
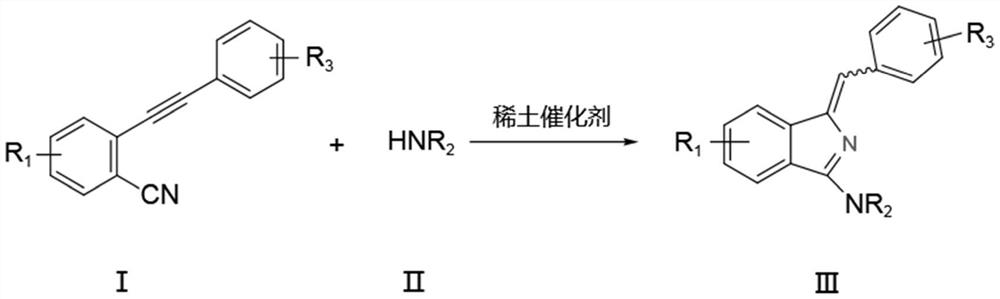
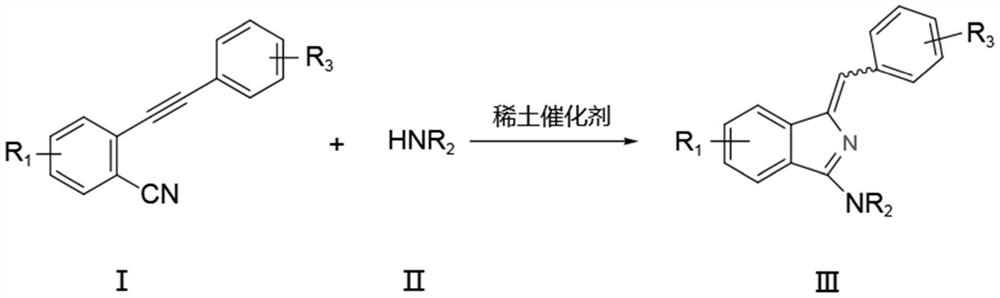
A kind of synthetic method of aminoisoindole derivative
A technology for indole derivatives and synthesis methods, applied in the field of organic synthesis chemistry, can solve the problems of poor step economy, harsh reaction temperature, incompatibility of secondary aryl amines and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of Z-1-benzylidene-3-(1-pyrrolidinyl)-isoindole, the structural formula is as follows:
[0036]
[0037] Under nitrogen protection, add raw material 2-phenylethynyl benzonitrile (0.5mmol), tetrahydropyrrole (0.6mmol) and catalyst La[N(SiMe 3 ) 2 ] 3 (5mol%), toluene (3mL), reacted at 25°C for 6h, and the isolated yield of the product was 98%.
[0038] 1 H NMR (CDCl 3 ,400MHz,ppm):δ8.29(d,J=8.0Hz,2H),7.80(d,J=8.0Hz,1H),7.71(d,J=7.6Hz,1H),7.43-7.38(m, 3H), 7.31(t, J=7.6Hz, 1H), 7.22(t, J=7.6Hz, 1H), 6.62(s, 1H), 3.99(t, 4H), 2.07(s, 4H). 13 C NMR (CDCl 3 ,100MHz,ppm): δ164.0,148.5,145.0,137.8,132.1,130.4,128.4,126.9,126.5,122.2,119.9,112.3,49.7.48.1,26.7,24.4.
[0039] The preparation of E-1-benzylidene-3-(1-pyrrolidinyl)-isoindole, the structural formula is as follows:
[0040]
[0041] Under nitrogen protection, add raw material 2-phenylethynyl benzonitrile (0.5mmol), tetrahydropyrrole (0.6mmol) and catalyst La[N(SiMe 3 ) 2 ] 3 (5mol%), ...
Embodiment 2
[0044] The preparation of Z-1-benzylidene-N-methyl-N-phenyl-1H-isoindol-3-amine, the structural formula is as follows:
[0045]
[0046] Under nitrogen protection, add raw material 2-phenylethynyl benzonitrile (0.5mmol), nitrogen methyl aniline (0.6mmol) and catalyst La[N(SiMe 3 ) 2 ] 3 (5mol%), toluene (3mL), reacted at 25°C for 6h, and the isolated yield of the product was 94%.
[0047] 1 H NMR (CDCl 3,500MHz,ppm):δ8.22(d,J=7.5Hz,2H),7.66(d,J=7.5Hz,1H),7.37-7.31(m,5H),7.25(s,1H),7.20- 7.14(m,3H),6.86(t,J=7.5Hz,1H),6.66(s,1H),6.01(d,J=8.0Hz,1H),3.70(s,3H). 13 C NMR (CDCl 3 ,125MHz,ppm): δ165.3,147.8,145.2,144.7,137.4,131.7,130.8,129.7,128.5,128.1,127.7,127.5,127.2,126.6,123.0,119.5,114.9,41.9.
Embodiment 3
[0049] The preparation of Z-1-benzylidene-N-methyl-N-benzyl-1H-isoindol-3-amine, the structural formula is as follows:
[0050]
[0051] Under nitrogen protection, add raw material 2-phenylethynyl benzonitrile (0.5mmol), nitrogen methyl benzylamine (0.6mmol) and catalyst La[N(SiMe 3 ) 2 ] 3 (5mol%), toluene (3mL), react at 25°C for 6h, and the isolated yield of the product is 90%.
[0052] 1 H NMR (CDCl 3 ,500MHz,ppm):δ8.28(d,J=8.0Hz,2H),7.84(d,J=7.5Hz,1H),7.61(s,1H),7.42-7.36(m,7H),7.31( t,J=7.0Hz,1H),7.26-7.21(m,2H),6.69(s,1H),5.11(s,2H),3.48(s,3H). 13 C NMR (CDCl 3 ,125MHz,ppm): δ166.6,147.7,145.5,137.7,137.6,131.5,130.6,128.9,128.5,128.4,127.6,127.5,127.1,126.9,122.8,120.0,113.5,55.6,38.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com