Fluorescence probe, preparation method and application thereof
A technology of fluorescent probes and fluorescent dyes, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of limited fluorescence enhancement of probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
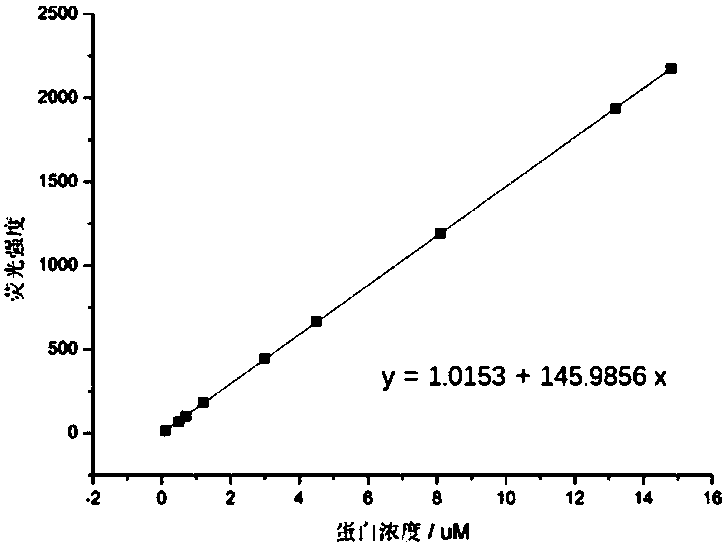
Image
Examples
Embodiment 1
[0144]
[0145] Compound 2:
[0146] Compound 1 (0.39g, 2mmol) was dissolved in a 100ml round bottom flask by adding 60ml of anhydrous methanol, adding malonic acid (0.25g, 2.4mmol), a catalytic amount of zinc chloride, and heating to reflux overnight in an oil bath under the protection of Ar. The next day, filter, remove part of the solvent by rotary evaporation, put the system in a refrigerator to cool and crystallize, filter, and rinse with cold ethanol three times to obtain 0.28 g of yellow crystals, with a yield of 61%. 1 H-NMR (400MHz, DMSO-d 6): δ=11.96(s, 1H), 7.82(d, 1H, J=16.0Hz), 7.49(d, 1H, J=7.2Hz), 6.69(d, 1H, J=7.2Hz), 6.52(s, 1H), 6.48 (d, 1H, J = 16.0 Hz), 6.44 (s, 1H).
[0147] Probe 1:
[0148] Compound 2 (0.23g, 1mmol) was added to a 25ml pear-shaped bottle, and compound 3 (refer to the published literature C.R.Jing, V.C.Cornish.ACS Chem.Biol., 2013, 8, 1704-1712.) (0.40g, 1.2mmol) , benzotriazol-1-yl-oxytripyrrolidinylphosphonium hexafluorophosphate...
Embodiment 2
[0150]
[0151] Compound 5:
[0152] Compound 4 (Refer to the method disclosed in the literature: X.P. Zhang. et al. ACS Macro Lett. 2016, 5, 229-233.) (0.49 g, 2 mmol) According to the method of compound 2, the yield was 89%. 1 H-NMR (400MHz, DMSO-d 6 ): δ=11.96(s, 1H), 7.78(d, 1H, J=16.0Hz), 7.51(d, 1H, J=7.2Hz), 6.71(d, 1H, J=7.2Hz), 6.49(d , 1H, J=16.0Hz), 6.52(s, 1H), 6.44(s, 1H), 3.41(t, 4H, J=8.2Hz), 1.21(d, 6H, J=8.2Hz).
[0153] Probe 2:
[0154] Referring to the synthesis method of probe 1, the yield was 92%. 1 H-NMR (400MHz, DMSO-d 6 ): δ=11.96(s, 1H), 7.78(d, 1H, J=16.0Hz), 7.51(d, 1H, J=7.2Hz), 7.28(s, 1H), 6.71(d, 1H, J= 7.2Hz), 6.64(s, 2H), 6.49(d, 1H, J=16.0Hz), 6.52(s, 1H), 6.44(s, 1H), 4.12(t, 2H, J=5.4Hz), 3.87 (s, 6H), 3.70(s, 2H), 3.41(t, 4H, J=8.2Hz), 3.30(t, 2H, J=6.0Hz), 2.08(m, 2H), 1.21(d, 6H, J = 8.2 Hz).
Embodiment 3
[0156]
[0157] Compound 7:
[0158] Compound 6 (refer to the method disclosed in the literature: WO 2006023821 (A2)) (0.53 g, 2 mmol) was synthesized according to the synthesis method of compound 2, and the yield was 66%. 1 H-NMR (400MHz, DMSO-d 6 ): δ=12.10(s, 1H), 7.76(d, 1H, J=16.0Hz), 7.46(d, 1H, J=9.2Hz), 6.84(s, 1H), 6.70(d, 1H, J= 16.0Hz), 5.99(d, 1H, J=9.2Hz), 3.26(m, 4H), 2.88(t, 2H, J=6.5Hz), 2.75(t, 2H, J=6.5Hz), 1.96(m , 4H).
[0159] Probe 3:
[0160] According to the synthesis method of probe 1, the yield was 88%. 1 H-NMR (400MHz, DMSO-d 6 ): δ=7.76(d, 1H, J=16.0Hz), 7.46(d, 1H, J=9.2Hz), 7.28(s, 1H), 6.84(s, 1H), 6.70(d, 1H, J= 16.0Hz), 6.64(s, 2H), 5.99(d, 1H, J=9.2Hz), 4.12(t, 2H, J=5.4Hz), 3.87(s, 6H), 3.70(s, 2H), 3.30 (t, 2H, J=6.0Hz), 3.26(m, 4H), 2.88(t, 2H, J=6.5Hz), 2.75(t, 2H, J=6.5Hz), 2.08(m, 2H), 1.96 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com