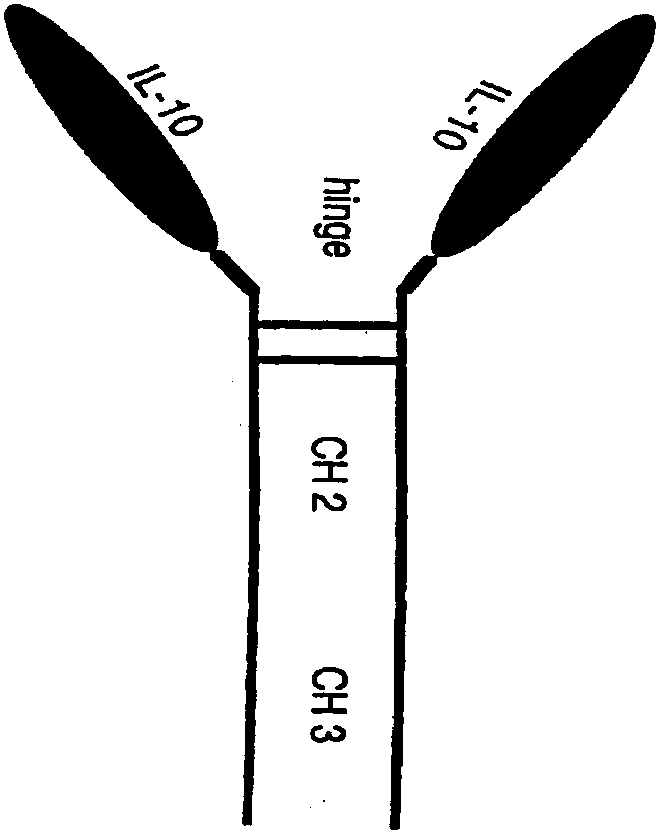
Human interleukin 10-Fc fusion protein, and coding gene and application thereof
A fusion protein, il10-fc technology, applied in human interleukin 10-Fc fusion protein and its coding gene and application field, can solve the problems of side effects of treatment, immunogenicity of Fc fusion protein, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: IL10-Fc fusion protein gene construction
[0053] SEQ ID NO: 3 was translated into a DNA sequence, and optimized according to the codon preference of CHO cells to obtain the expression sequence SEQ ID NO: 7 of the IL10-Fc fusion protein. Add NheI restriction site and kozac sequence (SEQ ID No.8) at the 5' end of the optimized sequence, and add stop codon and XhoI restriction site (SEQ ID No.9) at the 3' end to obtain fusion The complete expression cassette of the protein. The complete expression cassette sequence was artificially synthesized and inserted between the NheI and XhoI restriction sites of the pIRES plasmid to obtain the pIRES-IL10-Fc expression plasmid. After the plasmid was linearized, it was electrotransfected into CHO-s cells, and positive clones were screened by adding MSX.
Embodiment 2
[0054] Example 2: Expression and purification of IL10-Fc fusion protein
[0055] For the positive clones obtained in Example 1, after two rounds of limiting dilution, clones with better expression levels were screened out, and after expanded culture, they were inoculated into a 7L fermenter for fed-batch culture to express the target protein. Centrifuge at 4500rpm for 6min after fermentation, collect the supernatant, adjust the pH to 4.0, and store at 4°C.
[0056] The supernatant was first wrapped with a 10KDa ultrafiltration membrane and concentrated by ultrafiltration; then a preliminary affinity chromatography was performed with Mabselect Sure to collect the fusion protein. The mobile phase of affinity chromatography is: A1: 25mM PB+50mM Nacl, pH 7.0, B1: 20mM Gly, pH 3.0, B2: 20mM citric acid buffer, pH 3.0. The chromatographic column was first equilibrated with mobile phase A1. After loading the sample, the impurities were first eluted with mobile phase B1, and then the...
Embodiment 3
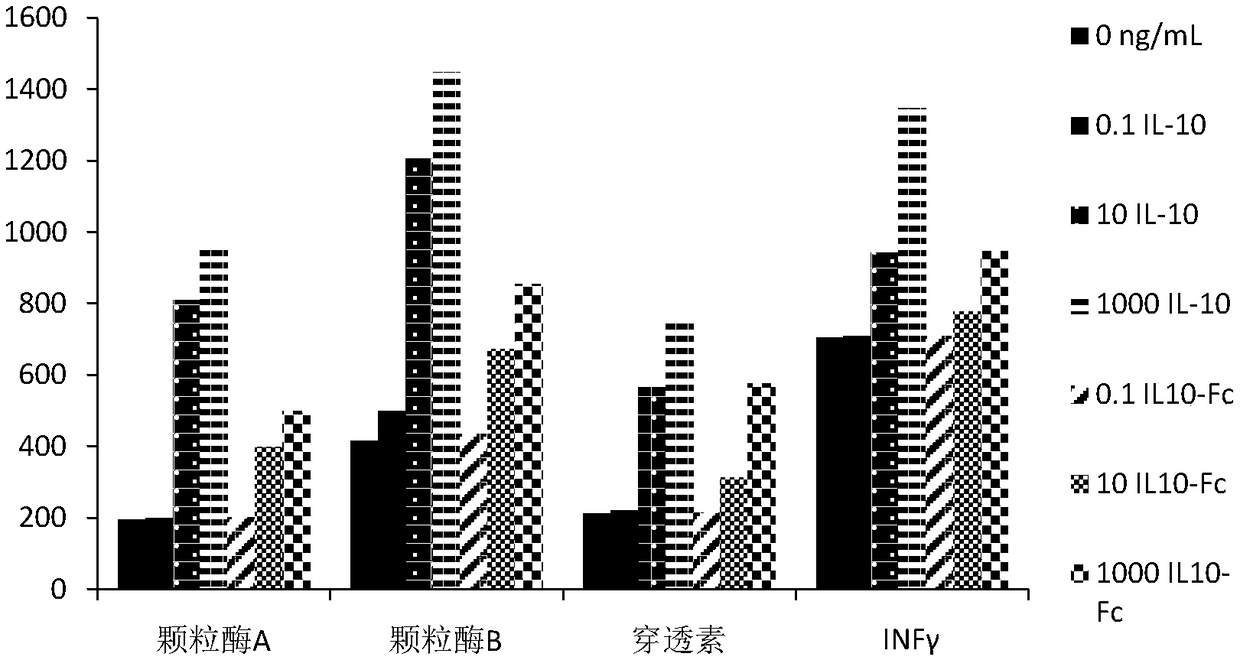
[0057] Example 3: In Vivo Half-Life Determination
[0058] Through tail vein injection, rhIL-10 (Rochy Hill Company) and the IL10-Fc fusion protein obtained in Example 2 were respectively injected into SD rats with an average body weight of about 200 g at a dose of 200 ng / Kg body weight). After injection, at different time points (0, 1, 2, 4, 6, 8, 12, 24, 36, 48, 60, 72, 96 hours) blood samples were collected by tail-cutting blood method, anticoagulated with heparin sodium, and The collected blood samples were centrifuged at 12000 g for 5 min to collect serum.
[0059] The human IL-10 ELISA kit (purchased from Bender Medsystem Company) was used to detect the content of the fusion protein in the blood samples according to the instructions, and the results were averaged. The results show that the elimination half-life of the IL10-Fc fusion protein prepared by the present invention is 22.6 hours in vivo, while the elimination half-life of human rhIL-10 is 4 hours after tail vei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com