Preparation and application of bionic medical fibroblast
A technology of byssin and protein, applied in the field of medical material preparation, can solve the problems of insolubility in neutral solution, application limitation, fast degradation speed, etc., to avoid the spread of terrestrial biological diseases, good adaptability and stability, biological good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Screening of Sbp5-2 protein
[0024] 1. Cloning of the whole gene of Sbp5-2
[0025] 1.1 Combined with the sequence information of mass spectrometry identification results, design RACE primers
[0026] Using Primer Premier 5 software to design primers for RACE (Rapid Amplification of cDNA Ends) Sbp-5-2
[0027] 3' RACE primer: TCCCAACAATGGAGTATGTGAAGATGCTG,
[0028] 5' RACE primer: GCCTAGTGTAACAGTCATTCTCCAGCCAAG;
[0029] 1.2 RNA extraction
[0030] Three patellofrerio scallops in the adaptation period (0-2h), the growth period of silk secretion (2-16h) and the balance period of silk secretion (16-24h) were dissected and their feet were taken according to the RNA was extracted by the method of Hu et al. (2006) and mixed in equal amounts. Using SMARTer™ RACE cDNA Amplification Kit (Clontech, CA, USA), according to the instructions, construct 5'RACE and 3'RACE libraries.
[0031] 1.3 Cloning the 3' and 5' ends of the target gene respectively
[0032] Add...
Embodiment 2
[0041] Example 2: Preparation of silk-forming fibers
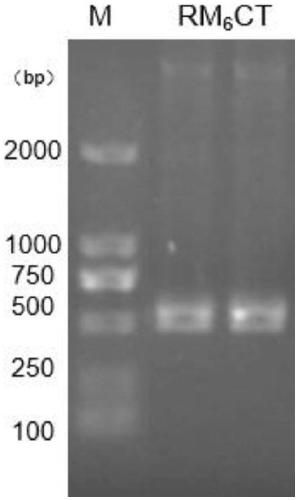
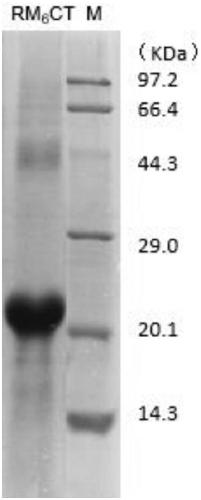
[0042] 1. RM 6 Clonal expression of CT
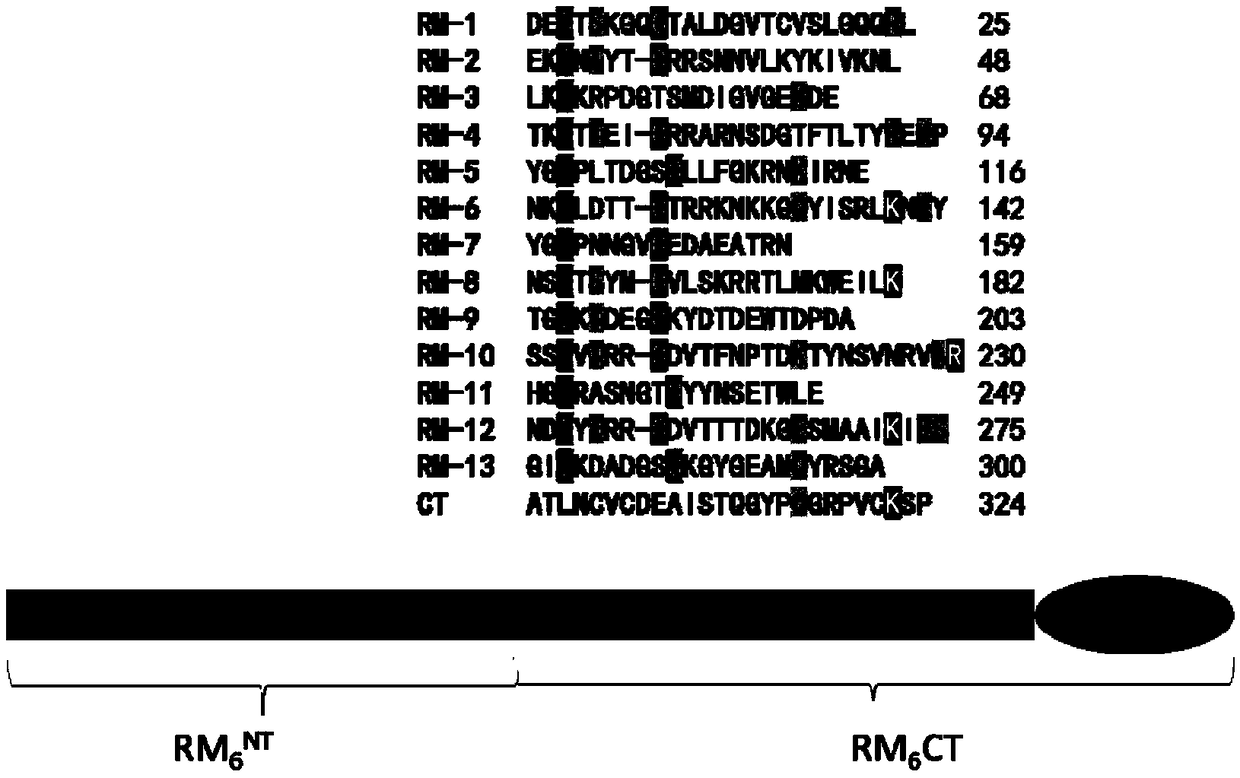
[0043] According to the amino acid sequence analysis of Sbp5-2, it was found that the part except the signal peptide sequence had multiple functional modules formed by paired cysteines, and a C-terminal (C Terminal) sequence different from the functional modules. The functional module contains a large number of hydrophilic amino acids, and the subclone RM of six functional modules connected after the C-terminal (C Terminal) sequence is selected by screening 6 CT (amino acid sequence number: 160-324) was purified and expressed. ( figure 1 ), the subclone RM 6 The sequence of CT is SEQ ID NO:2;
[0044]NKYYGCPNNGVCEDAEATRNNSCTSYMCVLSKRRTLMKWEILKTGCKTDEGCKYDTDEWTDPDASSCVTRRCDVTFNPTDKTYNSVNRVARHGCRASNGTCYYNSETWLENDCYTRRCDVTTTDKGESMAAIKIESGICKDADGSCKGYGEAMQYRSGAATLNCV;
[0045] 1.1RM 6 CT primer design
[0046] 5' Primer: GGGGTACCAACAGTTGCACCTCATATATGTGTA
[0047] 3' Primer: CCG...
Embodiment 3
[0070] Example 3: Properties of Filamented Fibers
[0071] 1. Characterization of the basic properties of silk-forming fibers:
[0072] 1) Material wet stability and water absorption detection
[0073] Soak the macroscopically formed wet fiber filaments (about 0.1g) in PBS solution, take them out at 0, 12, 24, 36, 48, 60, 72, 84, 96, and 108 hours, and blot the surface moisture with filter paper. Call the mass M wet . After freezing at -80°C, freeze-drying is carried out, and its mass is called M after freeze-drying. dry , do three parallel sets.
[0074] Swelling rate = (M wet -M dry ) / M dry ×100%.
[0075] Water absorption = (M wet -M dry ) / M wet ×100%.
[0076] Figure 5 for RM 6 The test results of CT protein fiber swelling rate (left picture) and water absorption rate (right picture); the test results show that it maintains a relatively stable swelling performance in a wet environment, and the material swelling rate maintains at about 400% within 108 hours. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com