Method for synthesizing sugammadex sodium through microwave reaction
A technology of sugammadex sodium and microwave reaction, which is applied in the field of microwave reaction synthesis of sugammadex sodium, can solve the problems of increasing the difficulty of industrial application and cumbersome processing process, and achieve good economy, simple follow-up purification and high product purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of Crude Sugammadex Sodium
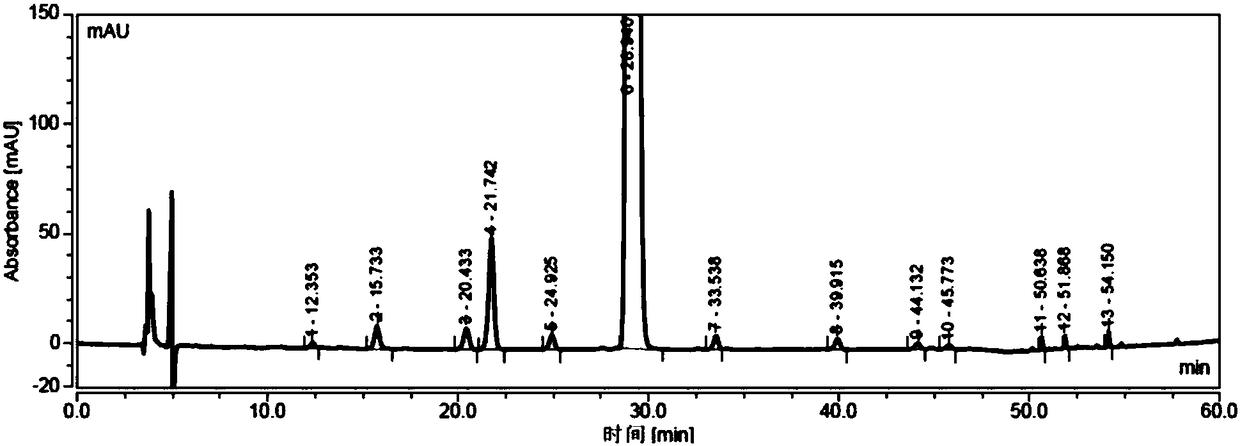
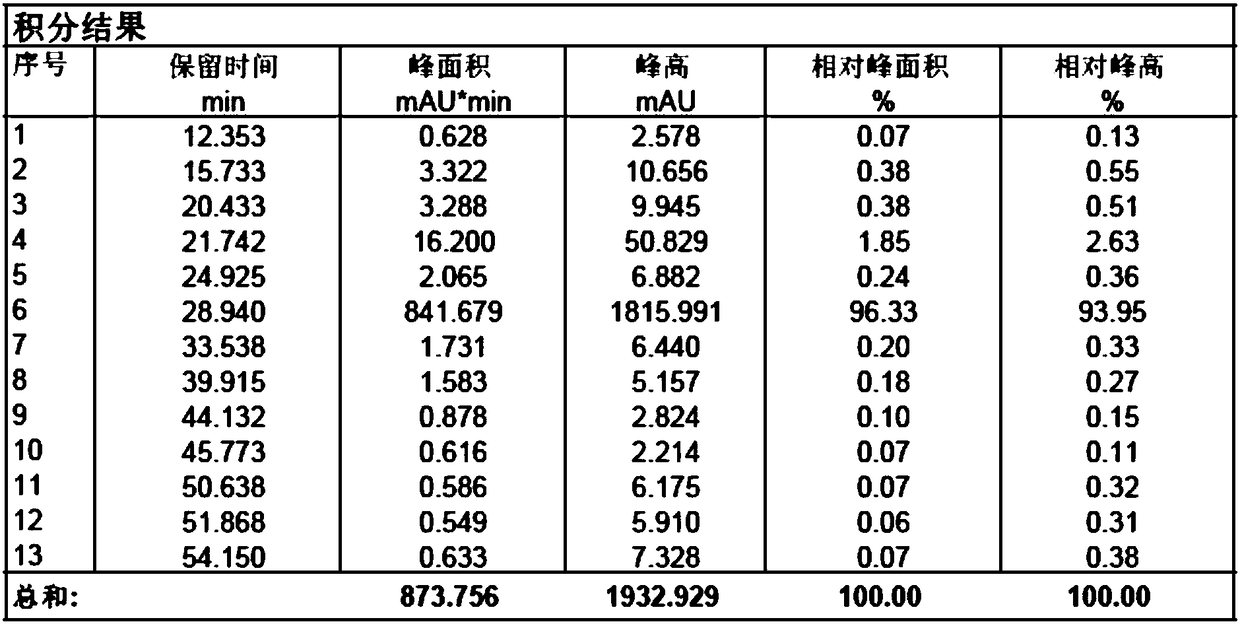
[0034] Add sodium hydroxide (23.5g, 588mmol) into the reaction flask, add 150ml water to dissolve completely; add dropwise 3-mercaptopropionic acid aqueous solution (39.0g, 368mmol, dissolve in 80ml water) to the flask under stirring, add 6-deoxy- 6-All-iodo-γ-cyclodextrin (40g, 18.4mmol) was added, placed in a microwave reactor, and reacted under 500w microwave radiation for 30 minutes; after the reaction was completed, cooled to room temperature, added 700mL DMF to crystallize while stirring, Filtrate, collect solid, vacuum-dry to obtain off-white solid 45g, purity is 98.33% (see attached Figure 3-4 ).
Embodiment 2
[0036] Purification of sugammadex sodium
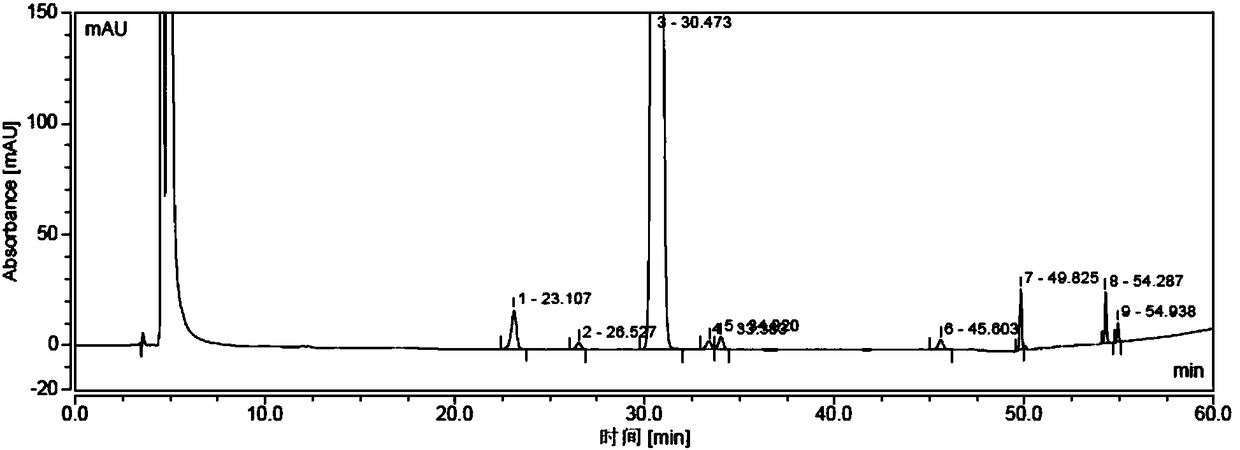
[0037] Under the protection of nitrogen, take 40 g of crude sugammadex sodium, dissolve it in a mixed solution of 100 ml of water and 600 ml of ethanol, stir and raise the temperature to 40°C, then cool down to room temperature, a white solid precipitates out, and is suction filtered to obtain 35 g of a white solid. This solid is detected by HPLC, and the purity is 99.82%, which meets the requirements of sugammadex sodium bulk drug (see attached Figure 5-6 ).
Embodiment 3
[0039] Synthesis of Crude Sugammadex Sodium
[0040] Sodium carbonate (15.9g, 150mmol) was put into the reaction flask, 75ml of water and 115ml of N,N-dimethylformamide were added; 3-mercaptopropionic acid aqueous solution (19.5g, 184mmol, dissolved in 80ml water), add 6-deoxy-6-periodo-γ-cyclodextrin (40g, 18.4mmol), after adding, put it in a microwave reactor, and react under 300w microwave radiation for 60 minutes; after the reaction is completed, cool to room temperature , add 400mL DMF to crystallize under stirring, filter, collect the solid, and vacuum dry to obtain 40g of off-white solid with a purity of 98.26%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com