Method for producing lithium chloride solution from lithium fluoride
A lithium fluoride and lithium chloride technology, applied in lithium halide, calcium/strontium/barium fluoride, calcium/strontium/barium halide, etc., can solve the problems of high material requirements, harsh operating environment and high consumption of hydrochloric acid , to achieve the effect of reducing the amount of hydrochloric acid, reducing environmental pollution and improving the operating environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
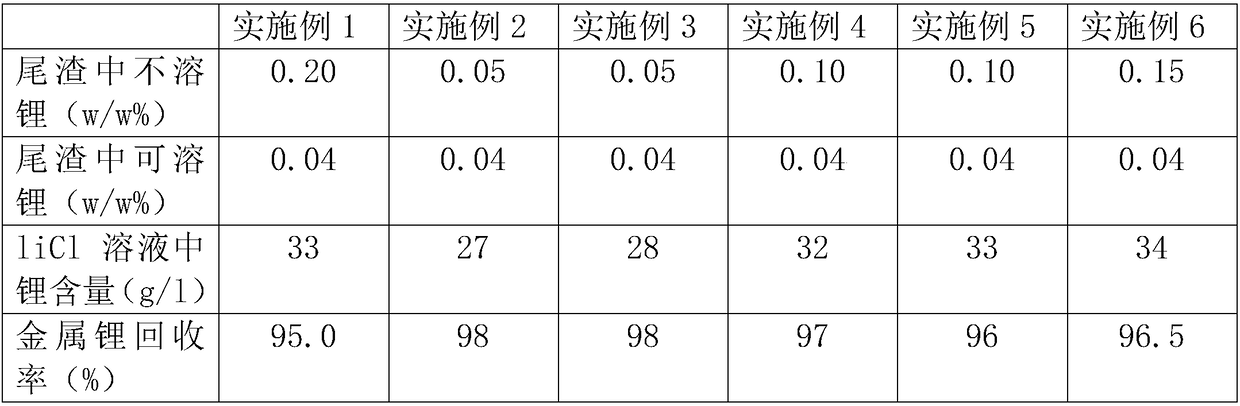
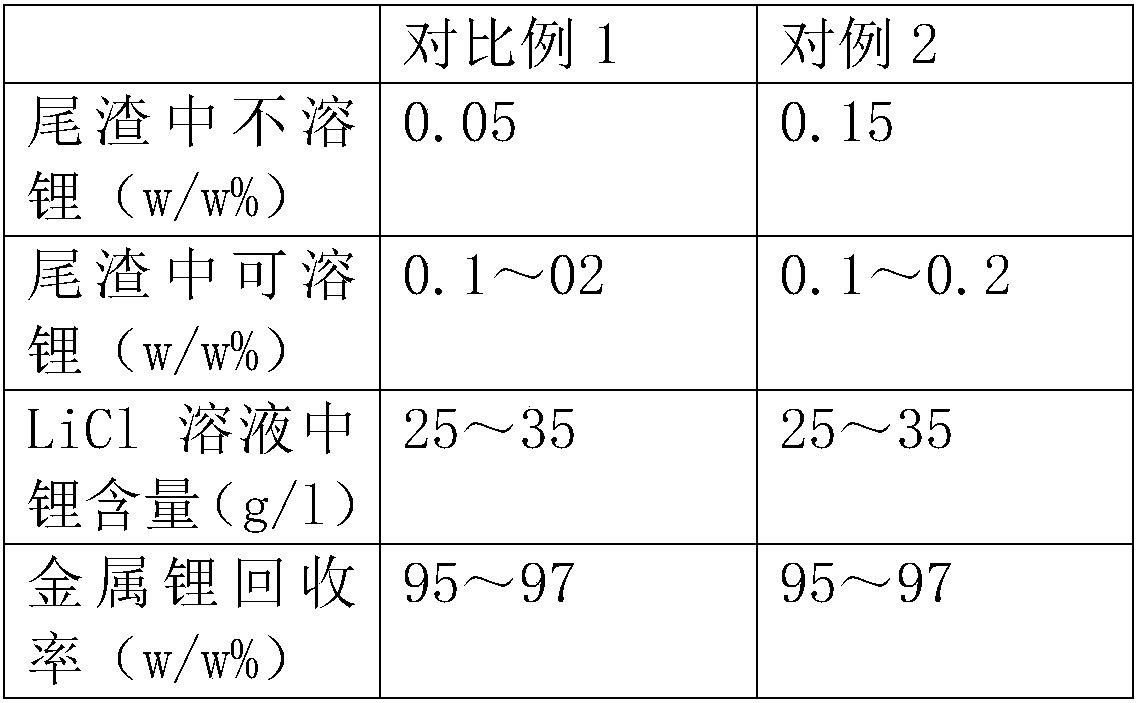
Embodiment 1
[0037] Add 2.5 square meters of production water to the reaction tank, start stirring, add lithium fluoride (100Kg of metal lithium), then add anhydrous calcium chloride (90% content) 1000Kg, add hydrochloric acid after adding calcium chloride to adjust the pH value to 1.5, heat to 85 degrees by steam, and keep it warm for 4 hours until the reaction is complete, then neutralize the pH to 6-8 with calcium hydroxide solution, filter to obtain lithium chloride solution. During the heat preservation process, the pH value remained unchanged, and the calcium ion content was 2.8 / l.
[0038] In order to better control the calcium ion content, the calcium ion content in the solution was detected after the incubation reaction for 30 minutes.
Embodiment 2
[0040] Add 3 square meters of production water to the reaction tank, start stirring, add lithium fluoride (100Kg of metal lithium), then add anhydrous calcium chloride (90% content) 1150Kg, add hydrochloric acid after adding calcium chloride to adjust the pH value to 2.0, heat to 85 degrees with steam, and keep warm for 4 hours until the reaction is complete, then neutralize the pH to 6-8 with calcium hydroxide solution, filter to obtain lithium chloride solution. During the heat preservation process, the pH value remained unchanged, and the calcium ion content was 19 / l.
[0041] In order to better control the calcium ion content, the calcium ion content in the solution was detected after the incubation reaction for 30 minutes.
Embodiment 3
[0043] Add 3 square meters of production water to the reaction tank, start stirring, add lithium fluoride (100Kg of metal lithium), then add anhydrous calcium chloride (90% content) 1250Kg, add hydrochloric acid after adding calcium chloride to adjust the pH value to 1.0, heated to 80 degrees with steam, and kept warm for 4 hours until the reaction was complete, then neutralized the pH to 6-8 with calcium hydroxide solution, filtered to obtain lithium chloride solution. During the heat preservation process, the pH value remained unchanged, and the calcium ion content was 33 / l.
[0044] In order to better control the calcium ion content, the calcium ion content in the solution was detected after the incubation reaction for 30 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com