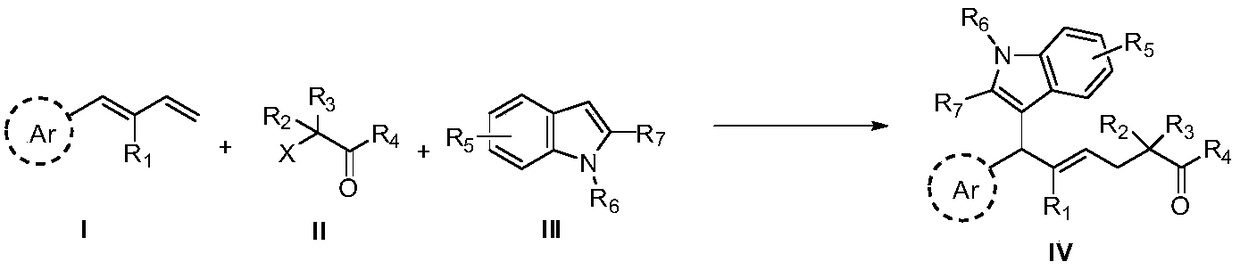
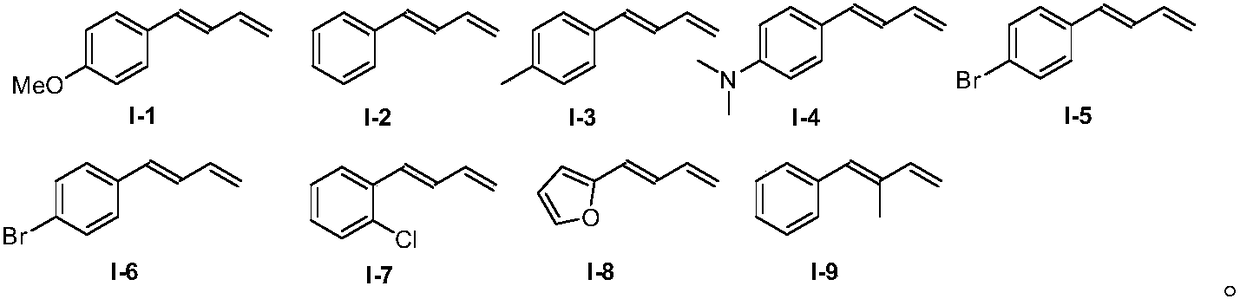
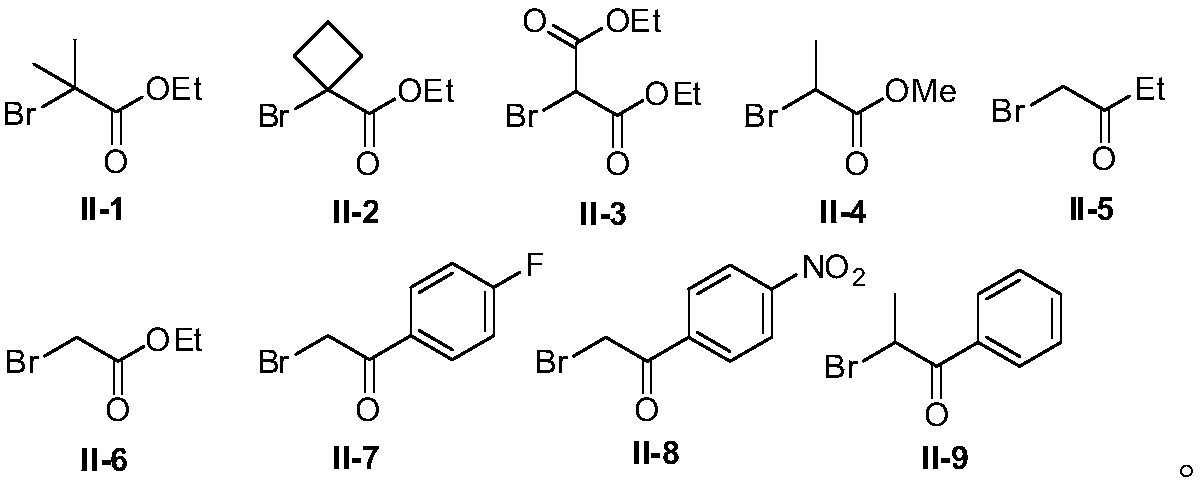
Method for preparing allyl indole compounds by 1,4-difunctionalization reaction of olefin
An allyl indole and compound technology, which is applied in the field of preparing allyl indole compounds by 1,4-difunctionalization reaction of olefins, can solve the problem of harsh process conditions, high reaction cost, difficult source of starting materials, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 19
[0066]
[0067] The compound of formula II-2 was used as the raw material, and the rest of the reaction raw materials, operations and parameters were the same as in Example 1 to obtain the target product IV-2 with a yield of 86%; 1 H NMR (500MHz, CDCl 3 )δ: 7.27(d, J=7.5Hz, 1H), 7.23(d, J=9.5Hz, 1H), 7.16(d, J=7.5Hz, 2H), 7.10(t, J=7.5Hz, 1H) ,6.94(t,J=7.3Hz,1H),6.78-6.76(m,2H),6.11-6.06(m,1H),5.44-5.38(m,1H),4.88(d,J=7.5Hz,1H ),4.06-3.99(m,2H),3.75(s,3H),3.65(s,3H),2.51(d,J=7.0Hz,2H),2.42-2.39(m,2H),2.31(s, 3H), 1.92-1.82(m, 4H), 1.15-1.12(m, 3H); 13 CNMR (125MHz, CDCl 3 )δ: 176.9, 157.7, 136.7, 135.9, 135.3, 133.1, 129.0, 126.8, 125.9, 120.3, 119.5, 118.5, 113.4, 112.7, 108.5, 60.2, 55.2, 47.4, 44.3, 40.6, 13, 41.5, 29. , 10.6.
Embodiment 20
[0069]
[0070] Using the compound of formula II-a as the raw material, the rest of the reaction raw materials, operations and parameters are the same as in Example 1 to obtain the target product IV-3 with a yield of 65%; 1 H NMR (500MHz, CDCl 3 )δ: 7.23(t, J=8.3Hz, 2H), 7.11(t, J=8.0Hz, 3H), 6.95(t, J=7.5Hz, 1H), 6.79(d, J=8.5Hz, 2H) ,6.19-6.14(m,1H),5.25-5.19(m,1H),4.92(d,J=6.0Hz,2H),4.65(d,J=6.0Hz,1H),4.62-4.57(m,2H ),3.78-3.73(m,5H),3.65(s,3H),2.49(d,J=7.5Hz,2H),2.30(s,3H); 13 C NMR (125MHz, CDCl 3 )δ: 157.8, 139.6, 136.7, 134.7, 133.3, 128.9, 126.5, 120.4, 119.9, 119.2, 118.7, 113.54, 111.5, 108.7, 93.7, 85.6, 70.2, 55.2, 44.0, 36.8, 26.5, 10.
Embodiment 21
[0072]
[0073] Using the compound of formula II-3 as the raw material, the rest of the reaction raw materials, operations and parameters were the same as in Example 1 to obtain the target product IV-4 with a yield of 66%; 1 H NMR (500MHz, CDCl 3 )δ: 7.27-7.22(m, 2H), 7.15(d, J=8.5Hz, 2H), 7.10(t, J=7.5Hz, 1H), 6.94(t, J=7.5Hz, 1H), 6.77( d,J=9.0Hz,2H),6.17-6.12(m,1H),5.48-5.42(m,1H),4.88(d,J=7.5Hz,1H),4.18-4.07(m,4H),3.75 (s,3H),3.65(s,3H),3.41(t,J=7.5Hz,1H),2.66(t,J=7.3Hz,2H),2.30(s,3H),1.22-1.16(m, 6H); 13 C NMR (125MHz, CDCl 3 )δ: 169.0, 157.7, 136.7, 135.7, 135.4, 133.1, 128.9, 126.7, 126.1, 120.3, 119.4, 118.6, 113.4, 112.4, 108.5, 61.3, 55.2, 52.1, 44.1, 31.7, 20, 10.6, 14.6
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com