Montelukast sodium preparation and preparation method thereof
A technology of montelukast sodium and its preparation, which is applied in the field of montelukast sodium preparation and its preparation, which can solve the problems of unseen and limited research and development, and achieve the effects of low water content, avoiding decomposition and deterioration, and relieving symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
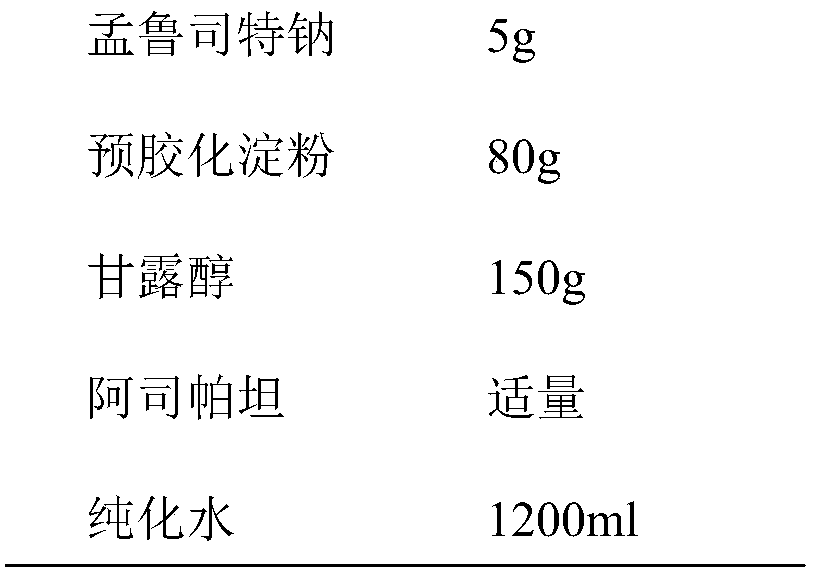
[0044] prescription:
[0045]
[0046]
[0047] Preparation:
[0048] 1) Micronizing the montelukast sodium bulk drug to obtain micronized montelukast sodium with a particle size of 80 μm;
[0049] 2) Put the pregelatinized starch in cold purified water and stir well until it is completely dissolved into a solution to obtain a pregelatinized starch solution;
[0050] 3) Weigh mannitol and micronized montelukast sodium according to the prescription amount, mix the two, then add to the pregelatinized starch solution obtained in step 2), stir well until the mannitol dissolves, and the montelukast The sodium is uniformly dispersed; then cooled under vacuum, and aspartame is added at the same time to obtain a suspension;
[0051] 4) Put the suspension obtained in step 3) into blister holes in divided doses, put it into a vacuum freeze-drying box for freeze-drying, and finally seal the drug-filled blister to obtain montelukast sodium freeze-dried orally disintegrating tablet...
Embodiment 2
[0053] prescription:
[0054]
[0055] Preparation:
[0056] 1) Micronizing the montelukast sodium bulk drug to obtain micronized montelukast sodium with a particle size of 100 μm;
[0057] 2) Put the pregelatinized starch in cold purified water and stir well until it is completely dissolved into a solution to obtain a pregelatinized starch solution;
[0058] 3) Weigh mannitol and micronized montelukast sodium according to the prescription amount, mix the two, then add to the pregelatinized starch solution obtained in step 2), stir well until the mannitol dissolves, and the montelukast The sodium is uniformly dispersed; then cooled under vacuum, and aspartame is added at the same time to obtain a suspension;
[0059] 4) Put the suspension obtained in step 3) into blister holes in divided doses, put it into a vacuum freeze-drying oven, cool down to minus 45°C, keep it for 2 hours, vacuumize, then gradually raise the temperature to 0°C, keep it for 2 hours, and then Gradua...
Embodiment 3
[0061] prescription:
[0062]
[0063] Preparation:
[0064] 1) Micronizing the montelukast sodium bulk drug to obtain micronized montelukast sodium with a particle size of 120 μm;
[0065] 2) Put the pregelatinized starch in cold purified water and stir well until it is completely dissolved into a solution to obtain a pregelatinized starch solution;
[0066] 3) Weigh mannitol and micronized montelukast sodium according to the prescription amount, mix the two, then add to the pregelatinized starch solution obtained in step 2), stir well until the mannitol dissolves, and the montelukast The sodium is uniformly dispersed; then cooled under vacuum, and aspartame is added at the same time to obtain a suspension;
[0067] 4) Put the suspension obtained in step 3) into blister holes in divided doses, put it into a vacuum freeze-drying oven, cool down to minus 45°C, keep it for 2 hours, vacuumize, then gradually raise the temperature to 0°C, keep it for 4 hours, and then Gradua...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com