Fluorinated derivative based on azobenzene and preparation method of fluorinated derivative
A technology of azobenzene derivatives and p-aminobenzoic acid, which is applied in the field of azobenzene-based fluorinated derivatives and its preparation, can solve the problems of limiting the application range of azobenzene, easy scattering of ultraviolet rays, and damage to organisms. Achieve the effects of reducing electron cloud density, excellent optical performance, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
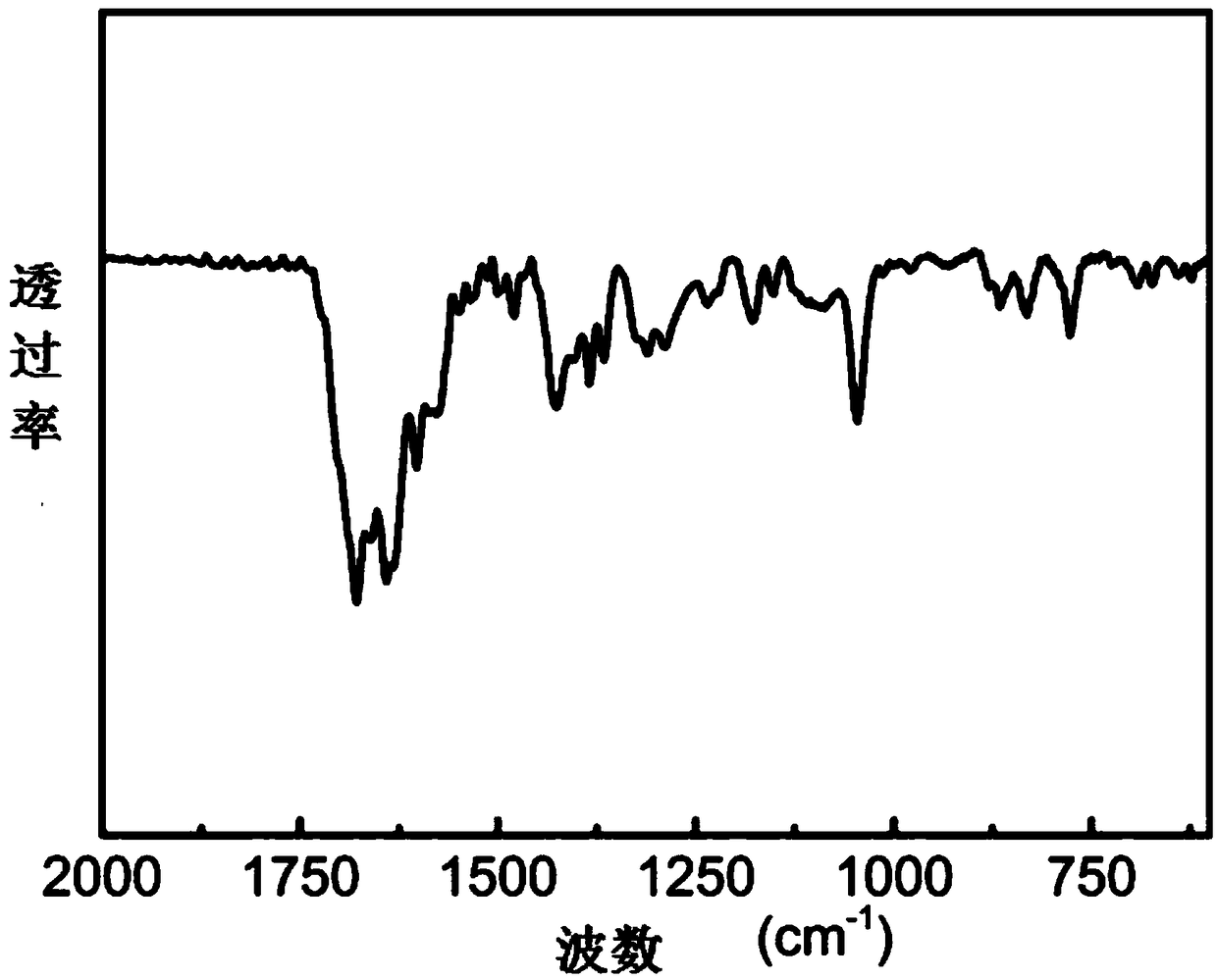
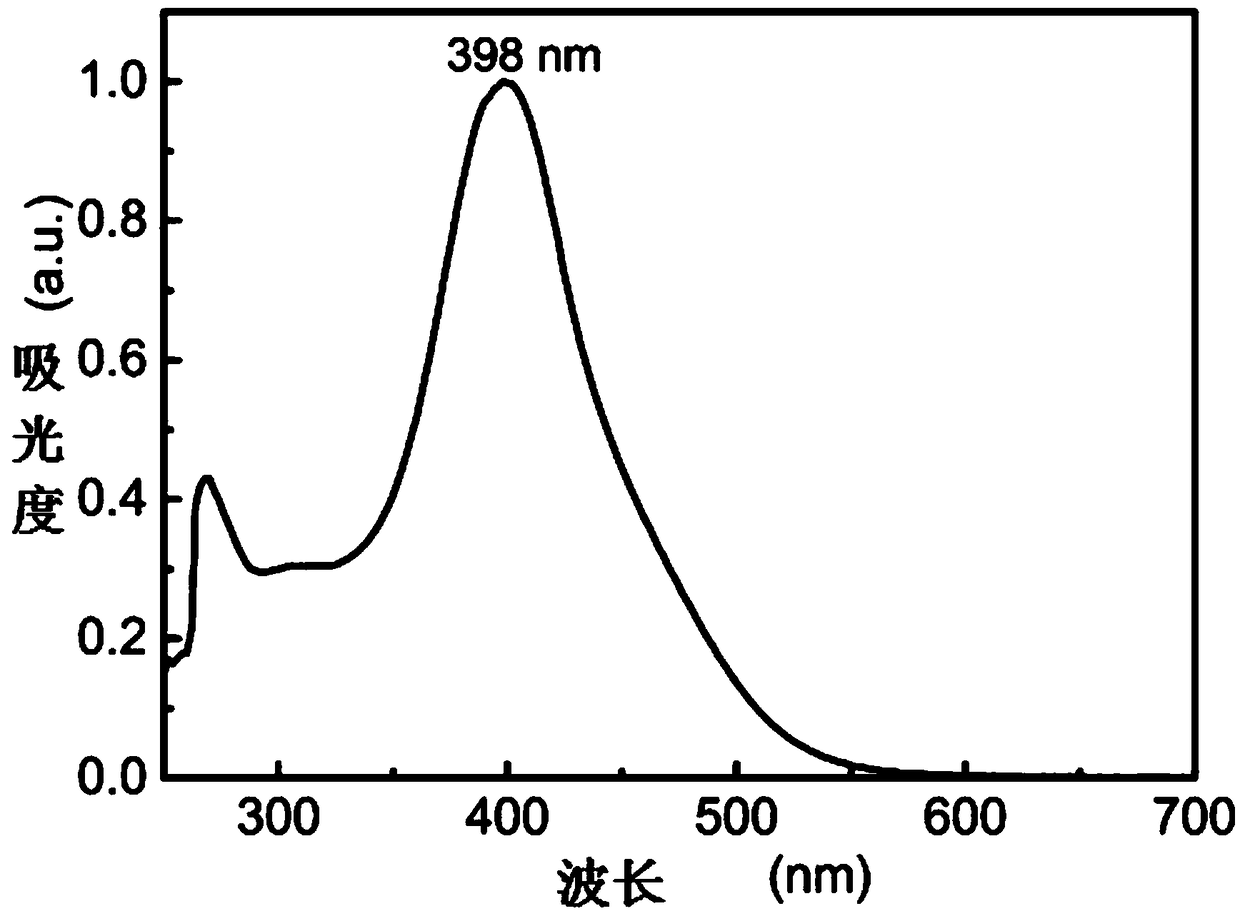
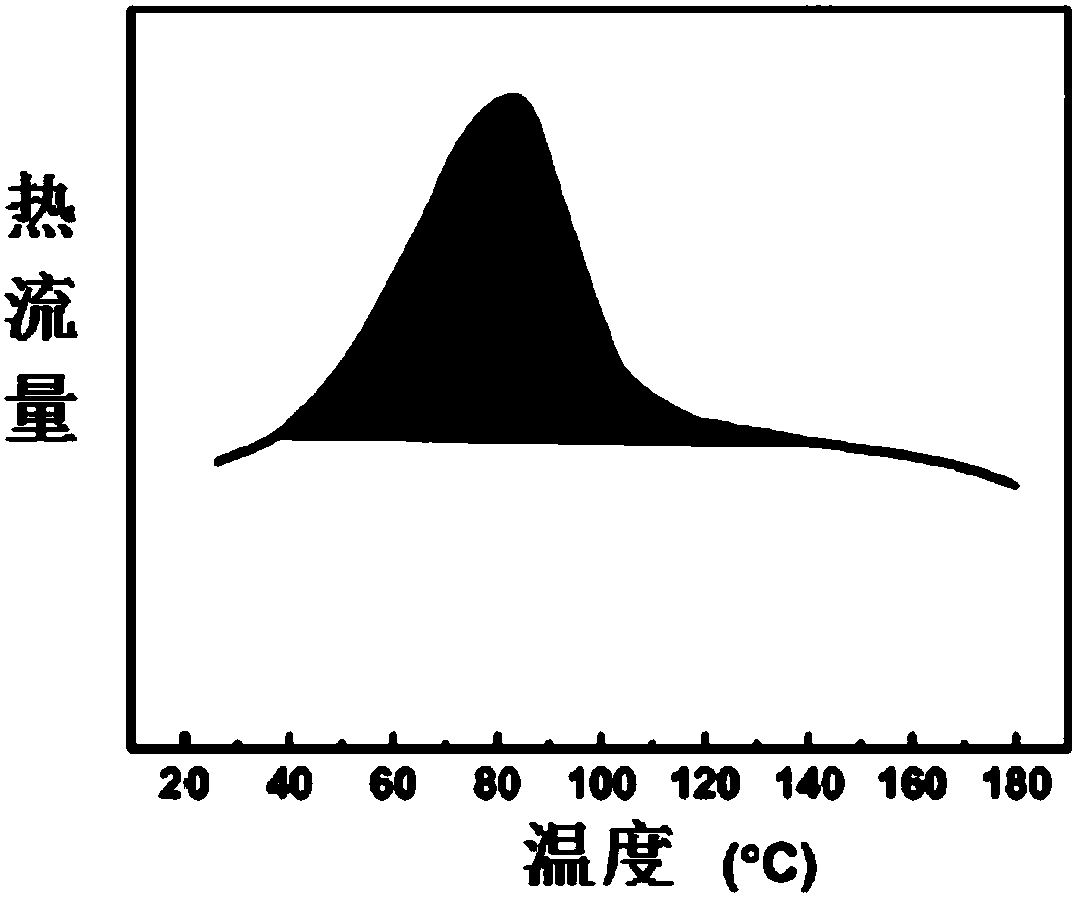
Image
Examples
Embodiment 1
[0030] 1) Preparation of diazonium salt: 1.37g of 4-aminobenzoic acid (10mmol) and 0.4g of NaOH (10mmol) were weighed and dissolved in 30mL of deionized water, and recorded as A. Weigh 0.76g NaNO 2 (11mmol) was dissolved in 5mL deionized water, and 10mL NaNO 2 Slowly added dropwise to A. After being completely dissolved, the solution was added dropwise to 40mL HCl (1molL -1 ), keep the temperature at 0-5°C, stir and react for 30 minutes to obtain a diazonium salt solution, and store it in an ice bath for later use.
[0031] 2) Coupling reaction: weigh 1.29g of 3,5-difluoroaniline (10mmol) (1:1 molar ratio to diazonium salt) and dissolve in HCl (1mol L -1 ), denoted as B; and the above-mentioned diazonium salt solution was slowly added dropwise to B and stirred.
[0032] 3) Adjust pH: prepare NaHCO 3 Saturated aqueous solution; add the aqueous solution dropwise to the above mixed solution to adjust the pH, and adjust the pH to 5-7; continue to stir for 10 hours under ice b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com